Abstract
Rat perivascular adipose tissue (PVAT) stores, takes up, and releases norepinephrine (NE; Ayala-Lopez et al 2014). We hypothesized that 3T3-L1 adipocytes would exhibit similar behaviors and, thus, could serve as a model for PVAT adipocytes. However, basal levels of NE were not detected in 3T3-L1 adipocytes. While incubation of 3T3-L1 adipocytes with exogenous NE increased their cellular NE content, the expression of mRNAs of several NE transporters [e.g. norepinephrine transporter (NET)] were not detected in these cells. Similarly, we observed expression of the vesicular monoamine transporter 1 (VMAT1) in 3T3-L1 adipocytes by qRT-PCR and immunostaining, but stimulation of the cells with tyramine (100 µM) did not cause a significant release of NE. These studies support that 3T3-L1 adipocytes are not an adequate model of perivascular adipocytes for studying NE handling.
Keywords: catecholamines, 3T3-L1 adipocytes, perivascular adipose tissue, obesity
Introduction
The rise of obesity and its comorbidity with cardiovascular disease has brought attention to the initial findings that perivascular adipose tissue (PVAT), the tissue surrounding the vasculature, alters arterial contraction (1). Specifically, the increased contraction in response to norepinephrine (NE) with removal of PVAT suggested that NE is being manipulated/handled by PVAT. This idea is validated by recent studies demonstrating that mesenteric PVAT takes up NE (2). This suggests that the absence of PVAT would contribute to an increased amount of NE causing contraction in vessels, and therefore could modify blood pressure. Furthermore, a functional reservoir of catecholamines, including NE and dopamine (DA), is present in PVAT. Upon stimulation with the indirect sympathomimetic tyramine, NE and DA were released from PVAT, independent of sympathetic nerves (3). This level of local control led us to question the presence of a non-neuronal adrenergic system within PVAT adipocytes. Outside of the evidence cited above, others have contributed to this idea. Specifically, the catecholamine synthesis enzymes tyrosine hydroxylase and phenylethanolamine N-methyltransferase are present in adipocytes isolated from rat mesenteric fat (4).
Adipocytes isolated from mesenteric PVAT have basal levels of NE, and NE has been imaged within mesenteric PVAT adipocytes through glyoxylic acid visualization and immunohistochemistry (3). Multiple PVATs contain catecholamines (3). One means by which NE could enter an adipocyte is through uptake. Organic cation transporter 3 (OCT3) is the main contributor to NE uptake into PVAT (2). The findings from these collective studies suggest the catecholamines present in mesenteric PVAT adipocytes exist through a combination of endogenous synthesis or taken up, supporting an adrenergic system within PVAT adipocytes.
This work raises the question of whether an adrenergic system is inherent to an adipocyte such that an adipocyte cell line could be used as a surrogate/model/comparator of PVAT adipocytes, as has been demonstrated in other studies (5, 6). Presently, we use the established cell line of the 3T3-L1 adipocytes. 3T3-L1 cells (murine origin), when differentiated, are similar to white adipocytes, though recent findings suggest that 3T3-L1 cells display features that represent multiple lineages (e.g. uncoupling protein-1 dependence; 7). Mesenteric PVAT is composed primarily of white adipose tissue. Because of this similar lineage, we hypothesized that 3T3-L1 adipocytes would 1) have endogenous stores of NE, 2) take up NE, and 3) release NE upon stimulation with tyramine, an indirect sympathomimetic that relies on plasma membrane transporters and vesicular amine transporters (VMAT) to cause release of intracellular stores of amines. If our findings are consistent with this hypothesis, then the 3T3-L1 adipocyte would be useful for greater ease of experimental manipulations (e.g. transfections) to study an inherent adrenergic system.
In initial experiments, catecholamines were measured via high-performance liquid chromatography (HPLC) in cells with and without exogenous NE incubation. Incubations without NE allowed for measurement of basal NE, while incubations with NE allowed testing of whether NE was taken up. Second, expression of canonical amine plasma membrane transporter genes, including genes for OCT3 and the norepinephrine transporter (NET), was tested using real-time PCR with species-specific positive controls for each gene and comparison to rat adipocytes isolated from PVAT. Finally, the ability of the 3T3-L1 adipocytes to store NE and the mechanism by which this could occur was tested by measuring mRNA expression and visualizing protein for vesicular monoamine transporter 1 (VMAT1). This was followed by measuring functional NE release by tyramine. This project is relevant because it could support the use of a more flexible cell system in investigation of systems that would reflect PVAT adipocytes. More generally, it is important to understand the role of adipocytes in the regulation of NE uptake in PVAT because of changes in PVAT function in obesity and hypertension that make PVAT less protective, producing reduced amounts of substances that typically relax a blood vessel (8–11). Such changes may impact how the blood vessel functions and, as such, may play a role in disease. It would be beneficial to have a more flexible system to model the PVAT adrenergic system.
Methods
Materials
Pargyline hydrochloride, Ro 41-0960, semicarbazide hydrochloride, tyramine hydrochloride and NE hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO, USA). Preadipocyte media reagents were Dulbecco’s modified Eagle medium (DMEM; Corning, cat. 90-013-PB), ascorbic acid (Sigma-Aldrich, cat. A4034), biotin (Sigma-Aldrich, cat. A4034), pantothenate (Sigma-Aldrich, cat. P5155), fetal bovine serum (Fisher Scientific, cat. SH3007304), antibiotic (Corning, cat. 30-004-Cl), L-glutamine (Gibco, cat. 25030-081), and sodium pyruvate (Corning, cat. 25-000-CI). Fetal bovine serum for adipocyte maintenance media was purchased from Corning (cat. MT35011CV).
Cell culture
3T3-L1 fibroblasts (Zenbio, Research Triangle Park, NC, USA; ATCC, Manassas, VA, USA) were seeded (2 × 104 cells/cm2) in a plastic T25 flask (Corning Inc. Corning, NY USA) and cultured in preadipocyte media [DMEM, 44.05 mM bicarbonate, 100 µM ascorbic acid, 33 µM biotin, 17 µM pantothenate, 10% calf serum, 1% antibiotic, 4 mM L-glutamine, 1 mM sodium pyruvate, 20 mM HEPES]. At 85–90% confluency, cells were passed using trypsin (0.25%, Sciencell, Carlsbad CA) and seeded in Falcon eight-well chamber slides (1.5 × 104 cells/cm2)(Corning Inc., Corning, USA), six-well (5 × 103 cells/cm2) and 96-well plates (6 × 103 cells/cm2) for uptake experiments, Oil Red O staining, and AdipoRed− assays, respectively. Cultures were maintained in a humidified incubator at 5% CO2 and 37°C. After two days at 100% confluency, 3T3-L1 fibroblasts were induced into adipocytes by adding 0.5 mM IBMX (Acros Organics, Fisher Scientific, Hampton, NH, USA) and 0.25 µM dexamethasone (Sigma-Aldrich, St. Louis, MO, USA) in addition to the maintenance media [which is preadipocyte media and 1 µg/mL insulin, 2 µM rosiglitazone (Adipogen, San Diego, CA, USA)]. After two days on differentiation media, cells were kept in maintenance media.
AdipoRed− assay
3T3-L1 preadipocytes and adipocytes ten days post induction were washed with phosphate buffered solution (PBS) once, then 200 µL of PBS were left in each well of a 96-well plate. Five µL of AdipoRed− reagent was added to each well. After ten minutes at RT and in the dark, fluorescence was measured at 485 nm and 572 nm for excitation and emission respectively on a Tecan Microplate Reader Infinite M1000 Pro (Life Sciences AG, Switzerland).
Oil Red O staining
Ten days post induction, 3T3-L1 adipocytes were stained for lipid. Media was removed, cells were washed 2 times with PBS, and PBS was removed. One mL of 10% neutral buffered formalin (Merck, Kenilworth, USA) was added for 30 minutes. Formalin was removed, cells were washed 2 times with PBS, then 1 mL of 60% isopropanol (diluted from 100% [Merck, Kenilworth, USA]) was added for 30 seconds. Isopropanol was removed, and 1 mL of Oil Red O working solution (6 mL Oil Red O stock [0.35 g Oil Red O in 100 ml isopropanol, filtered (0.2 micron) and stored at room temperature, Sigma, Cat# O-0625] and 4 mL of dH2O) was added for 10 minutes. Oil Red O solution was removed and cells were washed 4 times with water. Hematoxylin (Vector Laboratories, Burlingame, CA) was added for 30 seconds, removed, and cells were washed with water. Images were taken with an inverted microscope (DMi1 [Leica, Buffalo Grove, IL, USA) using Leica Application Suite (LAS).
Basal NE and NE uptake in 3T3-L1 adipocytes
NE was diluted into 1, 10, and 100 µM in PSS [containing (in mM) 130 NaCl, 4.7 KCl, 1.8 KH2PO4, 1.7 MgSO4·7H2O, 14.8 NaHCO3, 5.5 dextrose, 0.03 EDTA, and 1.6 CaCl2 (pH 7.2)] with 10 µM pargyline (in water; monoamine oxidase inhibitor), 1 µM Ro 41-0960 (in ethanol; catecholamine-O-methyltransferase inhibitor) and 1 mM semicarbazide (in water; semicarbazide-sensitive amine oxidase inhibitor). After three washes with PBS, vehicle or NE was added to 3T3-L1 adipocytes for 30 minutes at 37°C. Adipocytes were washed five times with PBS, collected in tissue buffer (0.05 mM sodium phosphate and 0.03 mM citric acid buffer, pH 2.5, in 15% methanol), flash frozen with liquid nitrogen, and stored at −80°C overnight. Samples were thawed and sonicated for three seconds, then centrifuged at 18,000 g for 15 minutes at 4°C. The supernatant was transferred into new tubes for HPLC analysis (Coulochem III electrochemical detector, HR-80 reverse-phase column, Cat-A-Phase II mobile phase). The cell pellets were dissolved in 1.0 M NaOH and assayed for protein using a Bicinchoninic Acid Protein Assay Kit (catalog no. BCA1, Sigma-Aldrich).
Animals
Male Sprague-Dawley rats (225–275 g, Charles River, Indianapolis, IN USA) were used. All protocols were approved by the Institutional Animal Care and Use Committee of Michigan State University and followed the Guide for the Care and Use of Laboratory Animals (8th ed., 2011). Rats were anesthetized with pentobarbital (60–80 mg/kg ip) and tissues dissected.
Tissue Dissection and Adipocyte Separation
Mesenteric PVAT was dissected from around small mesenteric vessels in the mesenteric arcade with the use of stereomicroscope. To digest PVAT, it was added to 1 ml PSS with 1 mg/ml collagenase from Clostridium histolyticum type IA (catalog no. C9891, Sigma-Aldrich) and incubated at 37°C with slow rotation (1 h). PVAT was centrifuged at 200 g for 5 min, and the stromal vascular fraction (SVF), which pellets to the bottom, was transferred to a separate tube. Adipocytes and the SVF were washed three times with PSS and centrifuged at 200 g for 10 min, then flash frozen and stored at −80°C.
Transporter mRNA Expression (plasma membrane and VMAT)
Tissue was homogenized using an Omni Bead Rupter. RNA was extracted with the Quick RNA MiniPrep kit (catalog no. R1054, Zymo Research, Irving, CA), and purity (260/280 and 260/230 ratios ≥ 1.8) was assessed using a Nanodrop 2000C spectrophotometer (Thermoscientific, Wilmington, DE). mRNA was reverse transcribed with a High Capacity cDNA Reverse Transcription kit (Thermoscientific Wilmington, DE). RT-PCR was performed using Taqman PerFecta FastMix (Quanta Bioscience, Beverly, MA, USA) on the ABI 7500 Fast Real Time PCR system using the following parameters: 95°C for 20 s, 95°C for 1 s, and 60°C for 20 s for 40 cycles. Measures were normalized to beta actin. The following primers were purchased from ThermoFisher Scientific (Wilmington, DE, USA): ActB (Mm02619580_g1; Rn00667869_m1), Slc6a2 (Mm00436661_m1; Rn00580207_m1), Slc22a3 (Mm00488294_m1; Rn00570264_m1), Slc6a4 (Mm00439391_m1; Rn00564737_m1), Slc6a3 (Mm00553058_m1; Rn00562224_m1), Slc29a4 (Mm00525575_m1; Rn01453824_m1), Slc18a1 (Mm00461868_m1; Rn00461866_m1), Slc18a2 (Mm00553058_m1; Rn00564688_m1).
Immunofluorescence staining for VMAT1
Ten days post-induction, cells were fixed in 4% paraformaldehyde in PBS for 10 minutes at room temperature, rinsed with PBS, and permeabilized with 0.2% Triton X-100 in PBS for 5 minutes at room temperature. The Triton solution was removed and cells rinsed with PBS. The medullary region of paraffin-embedded mouse adrenal gland (MP-501- C57, Zyagen, San Diego, CA) was used as a positive control tissue. To de-wax, the slide was dipped twice in HistoChoice Clearing Agent (H103, Amresco, Solon, OH), four times in 100% 2-propanol, and twice in water, all for three minutes each. The tissue was subsequently submerged and microwaved in 1% Antigen Unmasking Solution in dH2O (H-330, Vector Laboratories, Burlingame, CA) for 30 seconds for antigen retrieval, then rinsed with dH2O. The two serial tissue sections were then encircled using an ImmEdge pen (H-400, Vector). Both 3T3-L1 adipocyte and adrenal sections were blocked with 1.5% species-specific blocking serum (goat serum, S-1000, Vector) in PBS for 60 minutes at room temperature in a humidified chamber. Samples were given blocking serum or blocking serum with primary antibody [(1:50), anti-vesicular monoamine transporter 1, rabbit polyclonal antibody, AB1597P, Millipore, Temecula, CA). Slides were incubated overnight at 4°C in the humidified chamber. Blocking serum and primary antibody were removed and samples were rinsed three times with PBS for 5 minutes each. All samples were then incubated in secondary antibody [(1:1000), Alexa Fluor 488 conjugate, goat anti-rabbit IgG, A11008, Molecular Probes, Eugene, OR) made in PBS, in the humidified chamber, for 30 minutes in a 37°C incubator. Samples were rinsed three times with PBS, for 5 minutes each and were mounted with ProLong Gold antifade reagent with DAPI (P36935, Molecular Probes). Coverslips were added and sealed with clear nail polish. Slides were imaged using an Olympus FluoView 1000 Filter-based Laser Scanning Confocal Microscope mounted on an Olympus IX81 automated inverted microscope platform at the Michigan State University Center for Advanced Microscopy.
Catecholamine release in 3T3-L1 adipocytes
Tyramine was diluted to 100 µM in PSS with the same inhibitors [10 µM pargyline (monoamine oxidase inhibitor), 1 µM Ro 41-0960 (catecholamine-O-methyltransferase inhibitor) and 1 mM semicarbazide (semicarbazide-sensitive amine oxidase inhibitor)]. NE (10 µM) was added to 3T3-L1 adipocytes on 8 well chamber slides (Corning, NY USA) for 30 minutes at 37°C. After washing five times with PBS, vehicle or 100 µM tyramine was added to the PSS for 30 minutes at 37°C. Buffer solution (outside of cells) was immediately collected after incubation. Cells were collected with tissue buffer, and HPLC was used for catecholamine analyses. Values of NE released in the buffer were normalized to the protein content of the cells from which NE was released.
Imaging and data analysis
Images taken of 3T3-L1 preadipocytes, adipocytes, after Oil Red O staining and with VMAT1 antibody were taken with the same contrast, brightness, and saturation, and were not altered after imaging. N values for basal NE, NE uptake and release experiments represent a separate experiment for a different pass of the same original cells from the vial shipped by the manufacturer. RT-PCR results are reported as cycle threshold (CT) values out of 40 cycles. A Kruskal-Wallis test, a non-parametric test, was used to determine significance for NE uptake. A t-test was used to determine significance for the NE release results.
Results
3T3-L1 adipocyte differentiation was successful
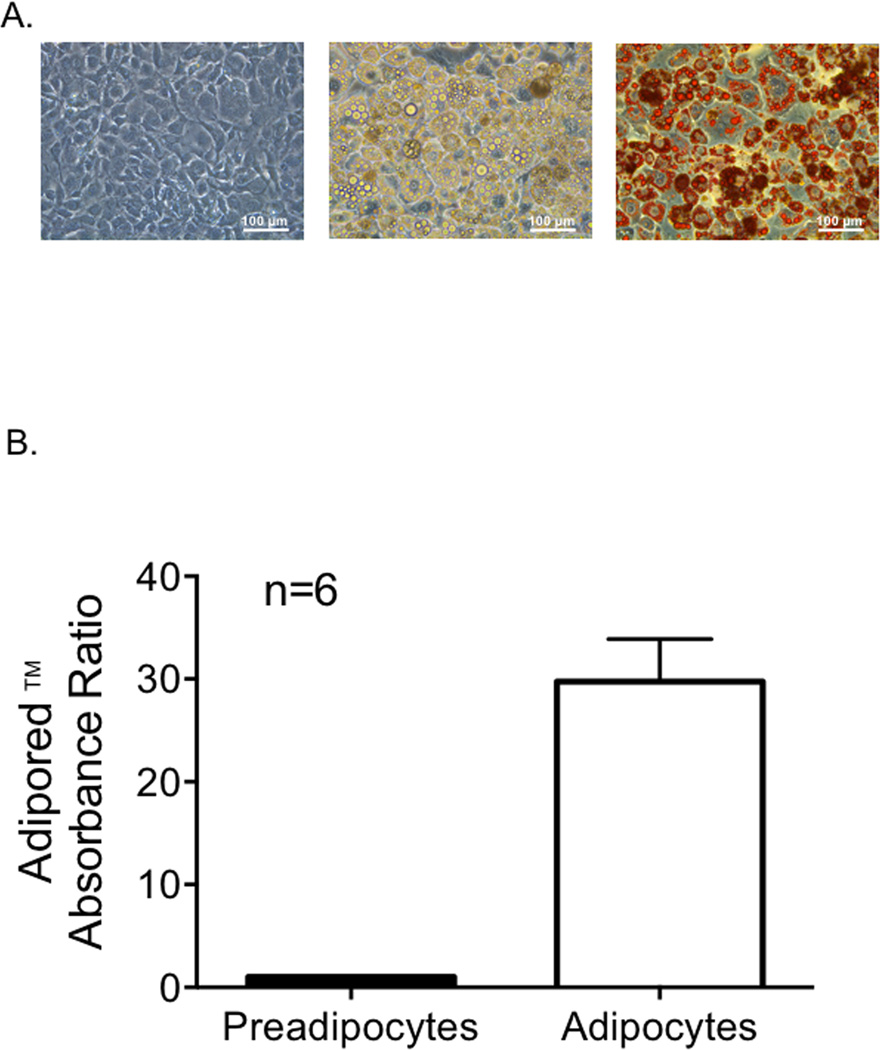
Figure 1A shows preadipocytes prior to induction (left), adipocytes ten days post-induction (middle), and adipocytes after Oil Red O staining (right). Lipid droplets are stained in red (figure 1A, right). The ratio of fluorescent signal between adipocytes and preadipocytes was 29.75 ± 9.21 (n=6) (figure 1B). Differentiated adipocytes should yield a ratio of at least ten (http://bio.lonza.com/uploads/tx_mwaxmarketingmaterial/Lonza_ManualsProductInstructions_AdipoRed_Assay_Reagent.pdf). These results indicate that our protocol for differentiating 3T3-L1 cells into adipocytes was successful.
Figure 1.
A. Left: 3T3-L1 preadipocytes before differentiation. Middle: adipocytes ten days post-induction. Right: adipocytes after Oil Red O staining, with lipid droplets stained in red. Preadipocytes were induced after two days at 100% confluency, to ensure growth arrest of the cells. Oil Red O staining was done 10 days post induction. Staining was done with six different passes of 3T3-L1 adipocytes. B. The ratio of absorbance between adipocytes and preadipocytes. The AdipoRed− reagent contains Nile Red, which has a distinct fluorescence when bound to triglyecerides. Bars represent mean ± SEM, n=6.
3T3-L1 adipocytes have low basal NE but concentrate exogenous NE
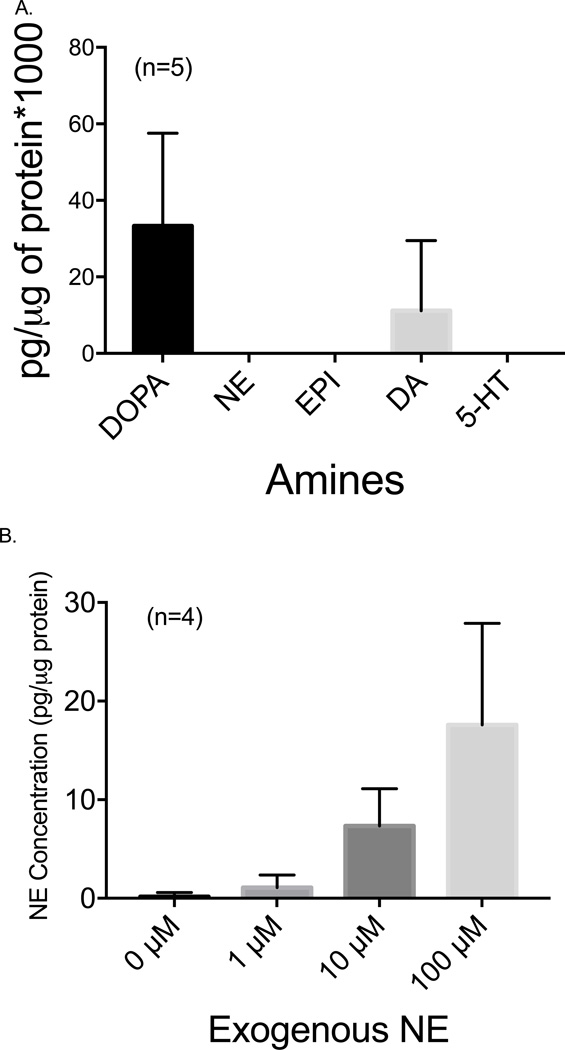
The 3T3-L1 adipocytes treated with vehicle in NE uptake experiments had undetectable levels of NE (figure 2A). However, both DOPA and DA were detected. After incubation with NE for 30 minutes, the cells were washed to remove excess NE, and NE in the cells was measured by HPLC. NE content increased from vehicle with the addition of 10 µM and 100 µM NE exposure (figure 2B). The ability of 3T3-L1 adipocytes to take up NE suggests a mechanism of transport exists for NE, and this was next investigated.
Figure 2.
A. Basal amine content in 3T3-L1 adipocytes after incubation with PSS for 30 minutes. Bars represent mean ± SEM, n=5. DOPA = 3,4-dihydroxyphenylalanine; NE = norepinephrine; E = epinephrine; DA = dopamine, 5-HT = 5-hydroxytryptamine. B. Uptake of NE by differentiated 3T3-L1 adipocytes. NE was added to adipocytes for 30 minutes at 37°C. Cells were washed multiple times to remove extracellular NE and then collected in tissue buffer. NE uptake was normalized to protein content. * p<0.05, *** p<0.001, vs. vehicle. Kruskal-Wallis test followed by Dunn’s Multiple Comparison Test was used to analyze the statistical significance of the data. Bars represent mean ± SEM, n=6.
3T3-L1 adipocytes do not express mRNA for canonical amine transporters
Messenger RNA for the plasma membrane transporters NET, OCT3, SERT, DAT and PMAT were not detected in 3T3-L1 adipocytes using mouse-specific primers. Mouse positive controls had the expected expressions of these transporter genes (table 1), validating our ability to detect the expression of the genes in the same species from which 3T3-L1 cells are derived. In comparison, isolated rat adipocytes from mesenteric PVAT had low detection of the mRNA for NET, SERT, and PMAT, and moderate detection of the mRNA for OCT3 (Ct value 26.13 ± 0.84, n=5) (table 1). Thus, 3T3-L1 adipocytes and mesenteric PVAT adipocytes are different in expression of these membrane transporter genes.
Table 1.
Plasma Membrane Transporter mRNA Expression
| 3T3-L1 Adipocytes (Mouse) | Rat Mesenteric PVAT Adipocytes | |||||
|---|---|---|---|---|---|---|
| Gene (Transporter) |
Samples Detected (Out of 4) |
Average Ct of Detected |
Ct of Positive Control (Mouse) |
Samples Detected (Out of 5) |
Average Ct of Detected |
Ct of Positive Control (Rat) |
|
Actb (Beta-Actin) |
4 | 21.26 ± 0.64 | Pons - 24.59 | 5 | 19.20 ± 0.73 | LC - 21.24 |
| Heart - 18.39 | Heart - 22.02 | |||||
| Brain - 18.64 | Adrenal - 17.18 | |||||
| Adrenal - 16.96 | ||||||
|
Slc6a2 (NET) |
1 | 37.45 | Pons - 18.45 | 5 | 37.36 ± 0.85 | LC - 30.64 |
|
Slc22a3 (OCT3) |
0 | ND | Heart - 26.94 | 5 | 26.13 ± 0.84 | Heart - 31.25 |
|
Slc6a4 (SERT) |
0 | ND | Brain - 27.53 | 5 | 35.00 ± 1.12 | LC - 27.74 |
|
Slc6a3 (DAT) |
0 | ND | Brain - 27.90 | 0 | ND | LC - 27.40 |
|
Slc29a4 (PMAT) |
1 | 37.41 | Brain - 26.97 | 5 | 32.49 ± 0.91 | LC - 28.61 |
ND = not detected; LC = locus ceruleus
Tyramine does not cause NE release from 3T3-L1 adipocytes
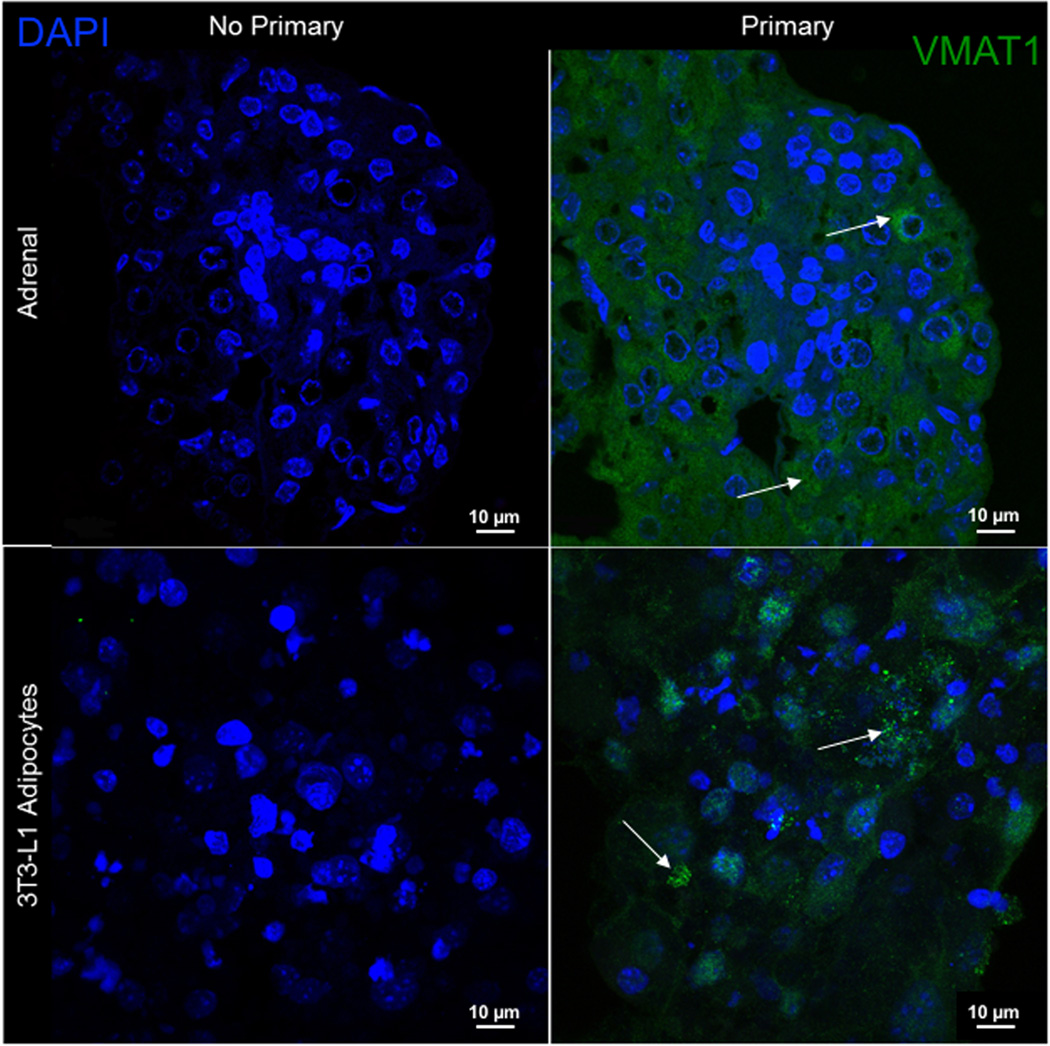
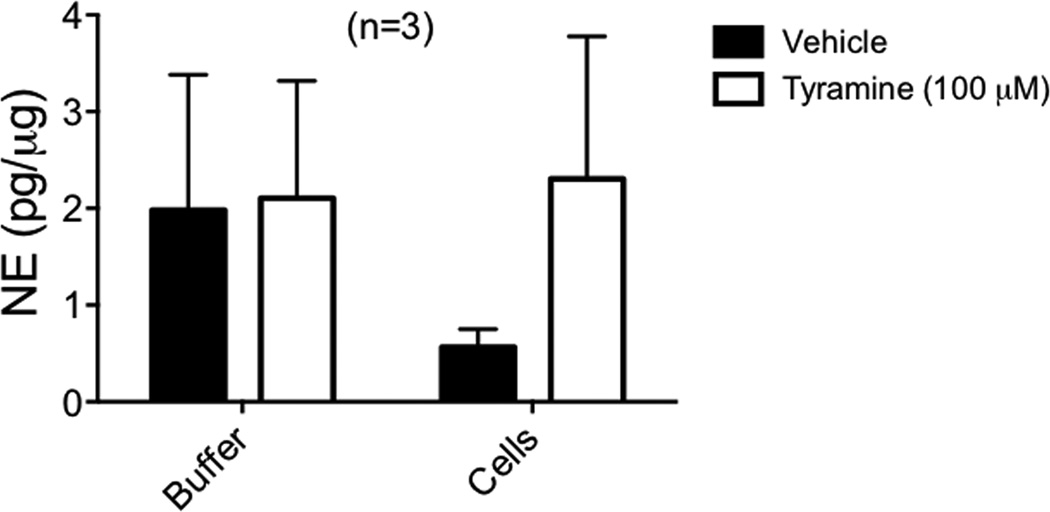
VMAT affords a concentration mechanism of amines for neurons, and thus is a logical choice to investigate in the 3T3-L1 adipocytes which concentrated NE. 3T3-L1 adipocytes positively expressed Slc18a1 (VMAT1) (Ct value of 34.04 ± 1.31). These results are shared here rather than Table 1, which is dedicated to plasma membrane transporters. Moreover, immunofluorescent staining showed that VMAT1 protein was expressed in the cytosol of the adipocyte (figure 3). The mouse adrenal was used as a species-specific positive control. However, the indirect sympathomimetic tyramine was not able to release NE from cells after they had the opportunity to concentrate NE through exogenous incubation of NE. This experiment was done with a time-dependent vehicle to account for basal NE release. Though basal amounts of released NE were observed (figure 4), there was no significant difference in NE release between vehicle and 100 µM tyramine (2.03 ± 1.53 pg/µg, 2.04 ± 1.45 pg/µg, n=3), suggesting that tyramine was ineffective in releasing intracellular stores of amines.
Figure 3.
Identification of VMAT1 protein in differentiated 3T3-L1 adipocytes. Images show the immunofluorescent staining of VMAT1 in mouse adrenals (top row; positive control) and 3T3-L1 adipocytes (bottom row). Nuclei are stained in blue (DAPI), VMAT1 is stained in green (FITC). Images were taken using a 90× magnification with a confocal microscope. Representative of five (5) differentiated passages.
Figure 4.
Inability of tyramine to release NE from 3T3-L1 adipocytes. After incubation with 10 µM NE for 30 minutes, 100 µM tyramine was added to 3T3-L1 adipocytes for 30 minutes. The solution collected immediately after tyramine incubation is the buffer solution. Cells were then collected in tissue buffer. Released NE measured through HPLC is normalized to the protein of the cells from which NE was released. Bars represent mean ± SEM. (n=3).
Conclusion
Our previous studies support that PVAT contains stores of catecholamines, NE is localized in the adipocyte cytoplasm of PVAT (3), and PVAT has the ability to release these stored catecholamines when challenged with tyramine. The present study tested the hypothesis that 3T3-L1 adipocytes would have similar abilities relative to catecholamine handling. Overall, our studies suggest that while there are similarities between the 3T3-L1 adipocytes and PVAT adipocytes, there is a remarkable difference in 1) basal levels of catecholamines; and 2) the plasma membrane transporters that handle catecholamines. This does not mean 3T3-L1 adipocytes are not at all useful for PVAT studies, but that the endpoints measured must be carefully compared to authentic PVAT/adipocytes before using the 3T3-L1 cells as a model.
Basal catecholamines
We began by asking whether catecholamines could be detected in the 3T3-L1 adipocytes. Catecholamines, internal to the cell, would be acquired through synthesis in the cell or through the culture media to which they are exposed. We could not detect NE in the cells, and only low levels of the precursors DOPA and DA were observed. We have not found other reports of either the presence or absence of catecholamines in this cell line, which may be because basal levels are undetectable. These findings make it unlikely that these cells have the ability to synthesize NE. We cannot exclude the possibility that the cells may have insufficient levels of the precursor for NE, tyrosine, but this idea conflicts with the presence of DOPA and DA that also use tyrosine as a precursor. By contrast, NE measured in rat PVAT and adipocytes isolated from PVAT was ~300 ng/g tissue or 300,000 pg/µg in comparison (2). The presence of NE in mesenteric fat has been observed by others (4). This is one of the ways in which 3T3-L1 adipocytes differ from PVAT adipocytes.
Uptake of NE in 3T3 adipocytes
The concentration-dependent uptake of NE by 3T3-L1 adipocytes contrasts with the absence of basal levels of NE. Qualitatively, this finding makes 3T3-L1 adipocytes similar to PVAT. However, the similarities stop there. In rat mesenteric PVAT adipocytes, NE uptake was mainly attributed to OCT3 (2). The absence of mRNA for OCT3, as well as other plasma membrane transporters of NE --NET, SERT, DAT, and PMAT-- suggests that NE is taken up by 3T3-L1 adipocytes via another mechanism. Alternatively, it is possible that the NE measured does not represent true NE uptake, but rather extracellular NE on the plasma membrane of 3T3-L1 adipocytes. However, cells (3T3-L1 adipocytes and PVAT adipocytes) were thoroughly washed prior to homogenization for measuring NE content. Adrenergic receptors themselves, once internalized, may provide a means to deliver NE into the cell. The functional transporter substrate ASP+ moved inside primary rat mesenteric adipocytes and could be located in the cytoplasm (2). This same tissue concentrated NE in a similar manner as to what was observed in 3T3-L1 adipocytes. As such, we can speculate that NE did move into the 3T3-L1 adipocyte, and the elevation of NE in the cell represents cellular concentration. However, without mRNA for any plasma membrane transporters recognized, we could not perform experiments using ASP+ in 3T3-L1 adipocytes. These findings raise a question that is difficult to answer, and that is whether plasma membrane transporter-independent mechanisms exist for NE concentration in a cell.
Storage and release of NE
The presence of VMAT1 protein in 3T3-L1 adipocytes suggested that it could be through VMAT1 that exogenous NE- shown to be elevated with exposure- would be stored. It is clear 3T3-L1 adipocytes are not exposed to high levels of NE in media given the undetectable basal levels of NE. The lack of measurable NE released after tyramine stimulation does make sense, and is also another way that 3T3-L1 cells are different from PVAT adipocytes. 3T3-L1 adipocytes do not express the mRNA for virtually every transporter that could take tyramine up into the cytoplasm. Thus, the most likely reason tyramine showed no effect is that it -- unlike NE -- could not get into the cell to be taken up by VMAT and release NE. By contrast, rat mesenteric PVAT expresses mRNA for NET, a transporter that allows tyramine entry into the cell (4). The lack of tyramine-induced release could also be because the NE concentrated by the 3T3-L1 cells is not intracellular, as described above. Thus, 3T3-L1 adipocytes cannot be used as a model for PVAT adipocytes when considering NE handling.
In addition to adipocytes, PVAT contains macrophages, fibroblasts, preadipocytes progenitors, nerves, etc, the collection of which is termed the stromal vascular fraction (SVF). Much of the work we have done previously is trying to separate out ‘where’ the adrenergic system is in PVAT. Can it wholly reside in the adipocyte? Are different elements in different parts of the PVAT? Using the 3T3-L1 cells was a move to try to help us answer these questions. We isolated adipocytes from mesenteric PVAT and showed the presence of NE in these cells in ways we could not in the 3T3-L1 cells (2,3). However, separation of adipocytes from PVAT is never perfect. There may cells from the SVF present in adipocyte fractions to which we could attribute NE measures. For example, macrophages contain NE amine transporters and tyrosine hydroxylase (13–15). Thus, while it is logical that the multicellular PVAT and 3T3-L1 adipocytes are not exactly the same, the 3T3-L1 adipocytes differ categorically from PVAT adipocytes in mechanisms of handling NE.
Limitations
All experiments were done using 3T3-L1 cells that were purchased. This makes the ‘n’ value different than what we typically use when creating a population from a group of animals, and thus there is inherent bias in narrowing the possible outcomes from such a discrete set of cells. Also, 3T3-L1 cells come from a mouse, not the rat we are studying relative to PVAT. We have measured catecholamines in mouse PVAT (data not shown), such that we know that mouse cells are similar to rat cells in this regard. Finally, it is possible that in using a culture system, we have disallowed important pre- and post-natal developmental transitions that could modify the genes expressed, such that the culture system is not a faithful reflection of what occurs in vivo (16).
Final Conclusions
The differences between 3T3-L1 adipocytes and rat mesenteric PVAT/adipocytes suggest that the cells neighboring adipocytes in PVAT may play a large role in the function of these adipocytes. 3T3-L1 adipocytes are not a good model of adipocytes for canonical catecholamine plasma membrane transporters, and caution should be used when applying the findings in this cell model to a larger scale.
Acknowledgments
This work was funded by NIH P01-HL70687 (SWW) and NIH F31 HL128035 (NAL). We acknowledge Dr. Andres Contreras (MSU) for help in adipocyte staining and growth.
Footnotes
Author contributions: Conception of the study (AI, NAL, SWW); execution of experiments (AI, NAL, MA), drafting of manuscript (AI, NAL, SWW), final reading of manuscript (AI, NAL, MA, SWW).
References
- 1.Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exper Journal of Hyper. 1991;13:277–296. doi: 10.3109/10641969109042063. [DOI] [PubMed] [Google Scholar]
- 2.Ayala-Lopez N, Jackson WF, Burnett R, Wilson JN, Thompson JM, Watts SW. Organic cation transporter 3 contributes to norepinephrine uptake into perivascular adipose tissue. Am J Physiol Heart Circ Physiol. 2015;309(11):H1904–H1914. doi: 10.1152/ajpheart.00308.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayala-Lopez N, Martini M, Jackson WF, Darios E, Burnett R, Seitz B, Fink GD, Watts SW. Perivascular adipose tissue contains functional catecholamines. Pharmacol Res Perspect. 2014 Jun;1(3):e00041. doi: 10.1002/prp2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vargovic P, Ukropec J, Laukova M, Cleary S, Manz B, Pacak K, Kvetnansky R. Adipocytes as a new source of catecholamine production. FEBS Lett. 2011;585:2279–2284. doi: 10.1016/j.febslet.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Takaoka M, Suzuki H, Shioda S, Sekikawa K, Saito Y, Nagai R, Sata M. Endovascular injury induces rapid phenotypic changes in perivascular adipose tissue. Arterioscler Thromb Vasc Biol. 2010;30:1576–1582. doi: 10.1161/ATVBAHA.110.207175. [DOI] [PubMed] [Google Scholar]
- 6.Barandier C, Montani JP, Yang Z. Mature adipocytes and perivascular adipose tissue stimulate vascular smooth muscle cell proliferation: effects of aging and obesity. Am J Physiol Heart Circ Physiol. 2005;289:H1807–H1813. doi: 10.1152/ajpheart.01259.2004. [DOI] [PubMed] [Google Scholar]
- 7.Morrison S, McGee SL. 3T3-L1 adipocytes display phenotypic characteristics of multiple adipocyte lineages. Adipocyte. 2015;4(4):295–302. doi: 10.1080/21623945.2015.1040612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aghamohammadzadeh R, Unwin RD, Greenstein AS, Heagerty AM. Effects of obesity on perivascular adipose tissue vasorelaxant function: nitric oxide, inflammation, and elevated systemic blood pressure. J Vasc Res. 2015;52:299–305. doi: 10.1159/000443885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ariemma F, D’Esposito V, Liquoro D, Oriente F, Cabaro S, Liotti A, Cimmino I, Longo M, Bequinot F, Formisano P, Valentino R. Low-Dose Bisphenol-A impairs adipogenesis and generates dysfunctional 3T3-L1 adipocytes. PLoS One. 2016;11(3):e0150762. doi: 10.1371/journal.pone.0150762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fesus G, Dubrovska G, Gorzelniak K, Kluge R, Huang Y, Luft FC, Gollasch M. Adiponectin is a novel humoral vasodilator. Cardiovasc Res. 2007;75:719–727. doi: 10.1016/j.cardiores.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 11.Galvez-Prieto B, Somoza B, Gil-Ortego M, Garcia-Prieto CF, de Las Heras AI, Gonzalez MC, Arribas S, Aranguez I, Bolbrinker J, Kreutz R, Ruiz-Gayo M, Fernandez-Alfonos MS. Anticontractile effect of perivascular adipose tissue and leptin are reduced in hypertension. Front Pharmacol. 2012 Jun 4;3:103. doi: 10.3389/fphar.2012.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-CT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 13.Brown SW, Meyers RT, Brennan KM, Rumble JM, Narasimhachari N, Perozzi EF, Ryan JJ, Stewart JK, Fischer-Stenger K. Catecholamines in a macrophage cell line. J Neuroimmunol. 2003;135:47–55. doi: 10.1016/s0165-5728(02)00435-6. [DOI] [PubMed] [Google Scholar]
- 14.Engler KL, Rudd ML, Ryan JJ, Stewart JK, Fischer-Stenger K. Autocrine actions of macrophage derived catecholamines on interleukin-1 beta. J Neuroimmunol. 2005;160:87–91. doi: 10.1016/j.jneuroim.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Rudd ML, Nicolas AN, Brown BL, Fischer-Stenger K, Stewart JK. Peritoneal macrophages express the serotonin transporter. J Neuroimmunol. 2005;159:113–118. doi: 10.1016/j.jneuroim.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Birsoy K, Berry R, Wang T, Ceyhan O, Tavazoie S, Friendman JM, Rodeheffer MS. Analysis of gene networks in white adipose tissue development reveals a role for ETS2 in adipogenesis. Development. 2011;138:4709–4719. doi: 10.1242/dev.067710. [DOI] [PMC free article] [PubMed] [Google Scholar]