Abstract
Background:
Here, we established the mouse models of chronic toxoplasmosis by T. gondii Tehran strain to provide a good understanding about defining the possible association between T. gondii exposure and learning and memory impairments. Moreover, as secondary objective of the present study, we hypothesized whether administration of an acetylcholinesterase (AChE) inhibitor could reduce learning and memory impairments induced by T. gondii infection.
Methods:
Twenty-four male BALB/c mice were used to establishment of latent toxoplasmosis. The animal model of Toxoplasma infection was established by the intraperitoneal inoculation of 20–25 tissue cysts from Tehran strain of T. gondii. Donepezil (2 mg/kg) an AChE inhibitor to treat Alzheimer disease was injected intraperitoneally once a day for two weeks starting from post-infection day 90. Morris water maze (MWM) task was used to assay spatial learning and short term spatial memory in all groups. One-way ANOVA with Tukey’s post-hoc test was used to assess differences between experimental groups. P<0.05 was considered statistically significant.
Results:
Toxoplasma infection impaired spatial leaning and short term spatial memory of the infected BALB/c mice, whereas donepezil, an AChE inhibitor, improved impairments induced by Toxoplasma infection.
Conclusion:
T. gondii infection through increasing AChE reduces the level of Acetylcholine (Ach) and consequently affects learning and memory activity in infected hosts, whereas, donepezil as an AChE inhibitor improves these impairments by restoring ACh levels at synapses of neurons in brain.
Keywords: Donepezil, Maze learning, Memory, Spatial learning, Toxoplasma gondii, Toxoplasmosis
Introduction
Toxoplasma gondii, as protozoan parasite, infects a broad spectrum of warm-blooded animals including humans. At present, approximately one-third of the world population has been infected with this parasite (1). T. gondii can cause infection in human via ingestion of tissue cysts or tachyzoites in raw or undercooked meat of infected animals, or ingestion of oocysts in the water or soil contaminated with feces of infected cats and congenitally (2). In human, T. gondii infection are normally asymptomatic or subclinical. However, the infection can cause serious symptoms including encephalitis, chorioretinitis, and systemic infections in immunocompromised individuals and abortion and stillbirth in pregnant women (3, 4). Commonly, after infection with T. gondii, tachyzoites run away from the immune system, cause the establishment of tissue cysts containing bradyzoites in various organs particularly in the central nervous system (CNS). At the time of chronic infection in CNS, T. gondii cysts can affect different biological functions of neuronal cells such as neurotransmitter synthesis, synapse organization, and signal direction (5, 6). Moreover, the epidemiological studies in humans as well as experimental investigations in animals demonstrated that chronic toxoplasmosis might be caused several neurological and behavioral disorders (7–12).
Previous studies indicate a main role of acetylcholine (ACh) in the regulation of cognitive and behavioral functions (13). On the other hand, T. gondii infection enhances the activity of acetylcholinesterase (AChE) as a membrane-bound enzyme which hydrolyses ACh (14, 15). Donepezil hydrochloride is a well-known anti-dementia drug that increases acetylcholine levels via inhibition of AChE and improve learning and memory impairments (16).
In this study, we established the mouse models of chronic T. gondii infection by T. gondii Tehran strain to provide a good understanding about defining the possible association between T. gondii exposure and learning and memory impairments. Moreover, as secondary objective of the current study, we hypothesized whether administration of an AChE inhibitor could reduce learning and memory impairments induced by T. gondii infection.
Materials and Methods
Ethical statement
The present study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Kerman University of Medical Science (No.93/20) and Kerman Neurosciences Research Center, Kerman, Iran. Moreover, all efforts were made to minimize suffering.
Animals
Twenty-four male BALB/c mice (6–8 weeks old) weighing from 20 to 25 g to establish animal model of T. gondii were obtained from the Animal Breeding Stock Facility of Razi Institute of Iran (Karaj, Iran). Animals were housed in a colony room of Kerman Neuro-sciences Research Center with a 12:12 h light/dark cycle at 21 ± 2°C and were handled according to standard protocols for the use of laboratory animals. BALB/c mice were randomly divided to three experimental groups (n=8 per group) for the all assays: T. gondii infection, uninfected (control), and T. gondii + donepezil group.
Parasite
In the present study, to establish the chronic toxoplasmosis, the Tehran strain of T. gondii was kindly provided by Prof. Keshavarz of Tehran University of Medical Sciences (Teharan, Iran). It was maintained by intraperitonealy inoculation of cysts (15–20 cysts) from brain tissue of infected BALB/c mice after 3 months. Cysts for the infection of BALB/c mice were isolated from the brain tissue of infected mice and the number of cysts was counted under a microscopy with a 10× objective.
Animal model of Toxoplasma infection
The animal model of Toxoplasma infection was established as described previously elsewhere (17). Brain homogenized suspension in saline was prepared from the mice infected with the tissue cysts of T. gondii three months earlier. Then, 0.5 ml of the brain suspension containing 20–25 tissue cysts was inoculated intraperitoneally to each of 8 male BALB/c mice. After 2 months, all the mice were tested for anti-T. gondii antibodies by serological tests.
Serological tests
In order to confirm Toxoplasma infection in tested mice, collected serum samples were examined for anti-T. gondii IgG antibody via the modified agglutination test (MAT) using a commercial kit (Toxoscreen DA, Biomérieux, Lyon, France) in accordance with the manufacturer’s instructions, and starting at a 1/20 dilution (18). Sera showing an agglutination titer of 1/20 or higher were considered positive and were end-titrated using 2-fold dilutions.
Treatment with AChE inhibitor
Donepezil hydrochloride (2 mg/kg, Sigma-Aldrich, USA) was dissolved in saline and was injected intraperitoneally once a day for two weeks starting from post-infection day 90.
Morris water maze (MWM) test
The MWM task was used to assay spatial learning and memory (19). The MWM consisted of a black circular swimming pool, painted with nontoxic materials black circular pool, 160 cm diameter, 80 cm height-filled with water maintained at room temperature to a depth of 40 cm. The pool was geographically divided into four quadrants of equal size and starting points were designated at each quadrant as N, S, E, and W. A square platform (10 cm diameter) was hidden just below (1.5 cm) the surface of the water in the center of the northeast quadrant. The experiments were carried out in a dimly light room with various and fixed extra maze geometric images (e.g., circles, squares or triangles) attached at different points on the walls around the maze. Performances were recorded by a smart video tracing system (Noldus Ethovision ® system, version 5, USA) and animals could be traced on the screen of a computer.
Spatial learning
In the spatial acquisition phase, the mice were allowed to find a submerged hidden platform during a 60-second-interval in four training trials (inter-trial interval = 60 s) repeated in three blocks (inter-block interval = 30 min). After finding the platform, the animals were allowed to rest on the platform for 20–30 s. The mice were dried with a towel and returned to their cages. After 20 to 30 s of rest, they were once again put in the chamber for the next trial. When mice did not find the platform within 60 s, the experimenter would put it on the platform. On each trial, mice were randomly released into the water from one of the four quadrants of the maze with their faces toward the wall of the quadrant where they were released. Each mouse had 4 different releasing points. Parameters such as latency and the traveled distance to find the platform were recorded in each trial.
Short-term spatial memory
Two hours after the acquisition phase, a probe test was performed to evaluate spatial memory retention. For the probe test, the platform was removed and each mouse was allowed to swim for 60 s. The time and distance spent in the target quadrant (quadrant 4) were analyzed as a measure of spatial memory retention.
Latency to visible platform and swimming speed
Following the probe trial, mice had to complete a visible platform test to determine any possibility of Toxoplasma infection and Aβ1–42 model interference with sensory and motor coordination or motivation. In this test, the ability of animals to escape to a visible platform was evaluated (the platform was raised 2 cm above the water level and was visible with aluminum foil).
Statistical analysis
Obtained results are expressed as the mean ± SEM. Data analysis was carried out by using SPSS statistical package version 17.0 (SPSS Inc., Chicago, IL, USA). One-way ANOVA with Tukey’s post-hoc test was used to assess differences between experimental groups (20). In addition, P<0.05 was considered statistically significant.
Results
Establishment of T. gondii infection
MAT showed that all of mice infected with T. gondii tissue cysts were seropositive for anti-T. gondii IgG antibody with agglutination titers of >1/20. Fig. 1 shows tissue cysts of T. gondii Tehran strain isolated from brain of infected mice.
Fig. 1:
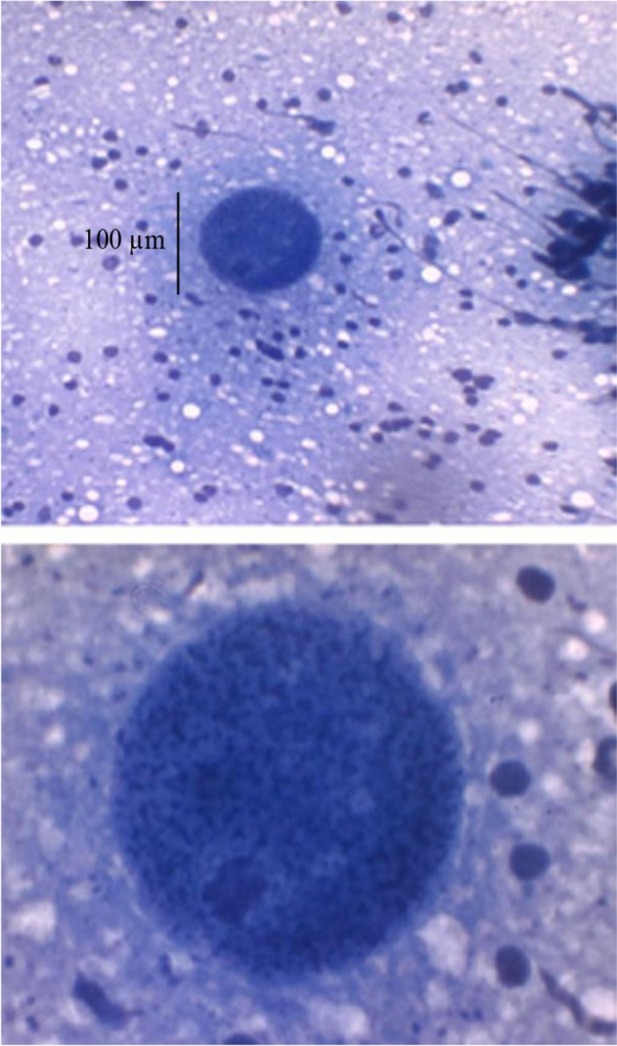
Tissue cysts of T. gondii Tehran strain In brain crushed smear of a mouse stained by Giemsa stain (at × 100 and × 400 magnification)
Spatial learning
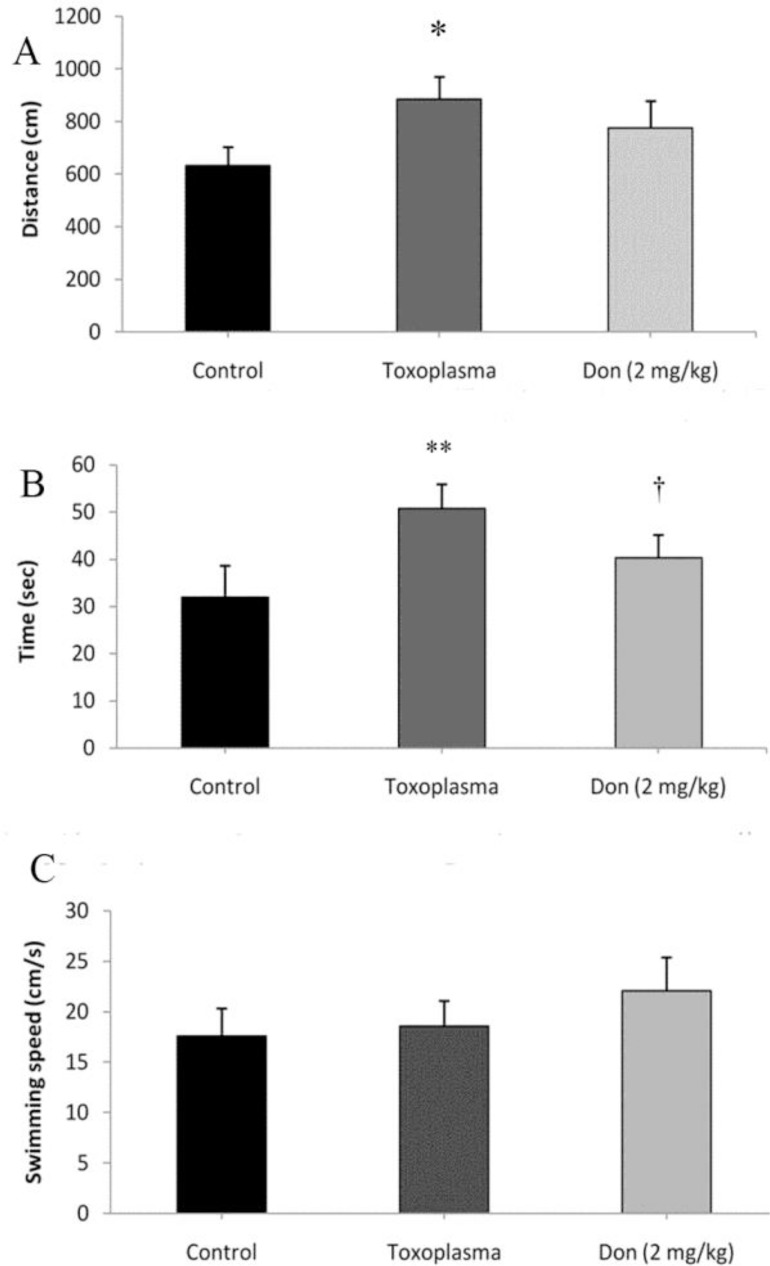
Distance traveled to reach the platform was significantly increased in the Toxoplasma infection group (P < 0.05) compared to the control group, indicating an impaired learning in Toxoplasma infected mice. Treatment with donepezil reduced the effect of T. gondii infection; the distance traveled to reach the platform was reduced in the T. gondii + donepezil mice in comparison with Toxoplasma infected mice (Fig. 2A).
Fig. 2:
Impaired learning observed in the Toxoplasma group compared to the control groups in Morris water maze task. Increased distance (A) and time spent (B) to reach the hidden platform were observed in the Toxoplasmagroup in compared with control group; while they were significantly decreased in mice treated by donepezil. There was no significant alteration in swimming speed of mice in all groups (C). * P < 0.05, ** P < 0.01, indicating the significant differences with the control group. † P<0.05 compared with the T. gondii group
As shown in Figure 2B, the escape latency of Toxoplasma group significantly (P < 0.01) increased in compared to the control group, whereas in the T. gondii infected mice treated with donepezil the escape latency significantly decreased (P < 0.05) compared to Toxoplasma group. Moreover, analysis of ANOVA indicated that there was no significant difference in the swimming speed among the all tested groups (Fig. 1C).
Short-term spatial memory
To assess short-term spatial memory retention, 2 h after the spatial learning phase, a probe test was carried out. The obtained results included the mean percentage (%) for time, distance (travel) and the number of crossing in the target quadrant.
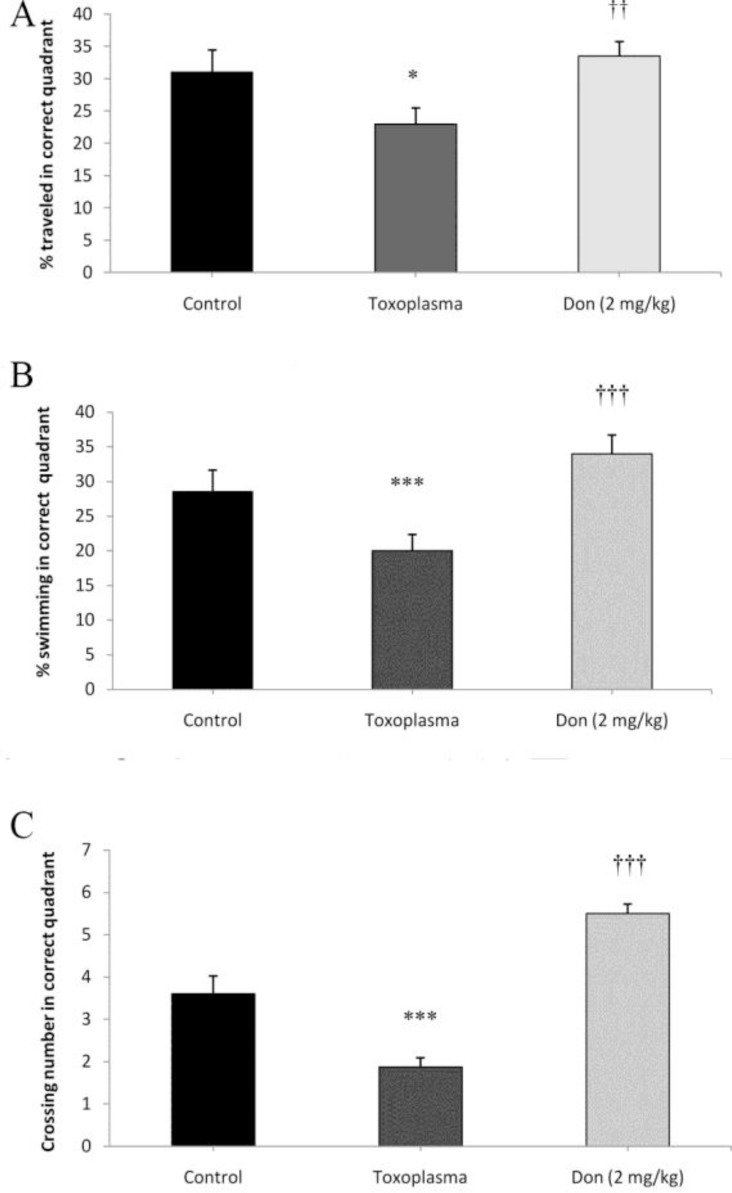
The findings revealed that the mice in the Toxoplasma group significantly (P < 0.05 for distance and P < 0.001 for time in the target quadrant) spent less distance and time in the target quadrant compared to the control groups (Figs. 3 A, B), which indicating short term memory impairment in the T. gondii infected mice. Obtained findings also revealed that treatment with donepezil counteracted the effect of T. gondii infection, because the Toxoplasma + donepezil mice spent more distance and time in the target quadrant compared to the Toxoplasma group (P < 0.01 for distance and P < 0.001 for time in the target quadrant). Analysis of ANOVA also showed that crossing number was significantly different in the Toxoplasma groups (P < 0.001) in comparison with control group (Fig. 3C), while in the T. gondii infected mice treated with donepezil the crossing number was significantly (P < 0.001) increased compared to Toxoplasma group.
Fig. 3:
The effects of Toxoplasma gondii infection and donepezil on spatial short term memory. The distance (A) and time (B) in the target quadrant decreased significantly in the Toxoplasma group compared to the control group. The number of crossing from the platform region was also significantly decreased in the Toxoplasma group compared to the control group. The distance, time, and the number of crossing were significantly increased in infected mice treated by donepezil. * P < 0.05, *** P < 0.001 indicating the significant differences with the control group. †† p<0.01 and ††† P<0.001 compared with the T. gondii group
Latency to visible platform and swimming speed
As shown in Table 1 mice in all groups had a similar escape latency and swimming speed in the MWM test, which indicating no significant differences between the groups in visual and motor functions.
Table 1:
Comparisons of swimming speed and latency to escape onto the visible platform in Morris water maze among groups using one-way analysis of variance (ANOVA) (the differences were not significant). Data are means ± S.E.M. (8 mice/group)
| Group | Swimming speed (cm/s) | Escape latency (s) |
|---|---|---|
| Control | 20.4 ± 3.6 | 18.8 ± 2.4 |
| Toxoplasma | 19.4 ± 1.8 | 20.1 ± 3.6 |
| Toxoplasma +Don * | 23.1 ± 2.3 | 21.2 ± 2.81 |
Donepezil 2 mg/kg/day/2w
Discussion
This study aimed to investigate the possible association between T. gondii exposure and learning and memory impairments, and also assessment of administration of donepezil, an AChE inhibitor to improve learning and memory impairments induced by T. gondii infection. T. gondii as a neurotropic protozoan parasite is considered as one of the most successful pathogens globally (21). Currently, there are several reports concerning the link of T. gondii infection with increased risk of some neurological disorders such as schizophrenia, personality disorders, obsessive-compulsive disorder, and Parkinson’s disease (7–12, 22).
We found that T. gondii infected mice represent remarkable impairments in the spatial learning and short time memory in MWM test compared to control group. Consistent with our results, Zhou et al. have reported that chronic toxoplasmosis induced by T. gondii Prugniaud strain impaired learning and memory functions in Kunming mice (23). In the infected rats, chronic toxoplasmosis lead to some neurocognitive disorders particularly memory impairment (24). In contrast, in latent toxoplasmosis, T. gondii in the CNS restrain neuronal degeneration as well as improves learning and memory disorders in mice with Alzheimer’s disease (25). This difference in the reported impacts of T. gondii infection on cognitive functions can be attributed to several factors including rodent species, route of infection, parasite strain, and dosage (24, 26, 27).
Changes in behavior observed during latent T. gondii infection can be the consequence of a range of indirect and/or direct effects. Whereas, indirect effects can involve immune response to chronic toxoplasmosis (28); the direct effects are probably due to the mechanical presence of the T. gondii in the brain or their effects or products on neuronal cells biology (29). Regarding direct effects of chronic toxoplasmosis, infection by T. gondii is able to affect cholinesterase activity and enhance the AChE levels in brain of infected mice (14, 15). ACh play a main role in the regulation of learning and memory functions (13). Today, the effective strategies to treat neurocognitive impairments designed to ameliorate the ACh activity through increase of level of Ach level via synthesis promoters as well as inhibitors of its metabolizing enzyme. Among the different approaches tried, the inhibition of AChE is the most successful one (30). To date, AChE inhibitors are widely used to improve the cognitive impairments such as learning and memory ones in patients with Alzheimer’s disease through enhance acetylcholine levels at synapses where the neurotransmitter has been evacuated in order to degeneration of neuronal cells (31).
Consistent with previous investigations, the obtained findings of the present study revealed a protective role for donepezil as an AChE inhibitor on learning and memory alterations induced by T. gondii. Donepezil at the dose of 2 mg/kg once a day for five consecutive days for two weeks improve spatial learning and memory deficits following traumatic brain injury independent of its effects on neurogenesis (16). Moreover, after treatment with donepezil a considerable improvement of memory as well as a dose-dependent depletion of amyloid-β (Aβ) was observed in brain of hAPP/PS1 mice (31). In gerbils, administratation donepezil at the dose of 5 mg/kg for 21 consecutive days reduced hippocampal neuro-degeneration and cognitive impairments after global cerebral ischemia (32). Our findings also exhibited that in MWM test donepezil resulted in an increase in swimming speed in treated mice. This suggests that one of the beneficial effects of donepezil on improvement of learning and memory in T. gondii infected mice which might be through improvement in motor activity as described elsewhere (16).
Conclusion
T. gondii infection through increasing AChE reduces the level of ACh and consequently affects learning and memory activity in infected hosts, whereas, donepezil as an AChE inhibitor improves these impairments by restoring ACh levels at synapses of neurons in brain. However, further studies are required to elucidate these mechanisms and other possible ones.
Acknowledgements
We would like to thank Dr. Amir Tavakli Kareshk to establish the T. gondii infection in BALB/c mice. The authors declared no conflict of interest in the present study.
References
- 1. Hill D, Dubey J. Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect. 2002; 8 (10): 634– 640. [DOI] [PubMed] [Google Scholar]
- 2. Fallahi S, Seyyed Tabaei SJ, Pournia Y, Zebardast N, Kazemi B. Comparison of loop-mediated isothermal amplification (LAMP) and nested-PCR assay targeting the RE and B1 gene for detection of Toxoplasma gondii in blood samples of children with leukaemia. Diagn Microbiol Infect Dis. 2014; 79 (3): 347– 354. [DOI] [PubMed] [Google Scholar]
- 3. Tavakoli Kareshk A, Keyhani A, Asadi A, Zia-Ali N, Mahmoudvand H, Mohammadi AR. Seroprevalence of Toxoplasma gondii infection among childbearing age women in the city Kerman, southeastern Iran. J Parasit Dis. DOI 10.1007/s12639-015-0724-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rostami A, Keshavarz H, Shojaee S, Mohebali M, Meamar AR. Frequency of Toxoplasma gondii in HIV positive patients from West of Iran by ELISA and PCR. Iran J Parasitol. 2014; 9 (4): 474– 81. [PMC free article] [PubMed] [Google Scholar]
- 5. Prandovszky E, Gaskell E, Martin H, Dubey JP, Webster JP, McConkey GA. The neurotropic parasite Toxoplasma gondii increase dopamine metabolism. PLoS One 2011; 6: e23866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gatkowska J, Wieczorek M, Dziadek B, Dzitko K, Dlugonska H. Sex-dependent neurotransmitter level changes in brains of Toxoplasma gondii infected mice. Exp Parasitol. 2013; 133 1: 1– 7. [DOI] [PubMed] [Google Scholar]
- 7. Celik T, Kamişli O, Babür C, Cevik MO, Oztuna D, Altinayar S. Is there a relationship between Toxoplasma gondii infection and idiopathic Parkinson’s disease? Scand J Infect Dis. 2010; 42( 8): 604– 8. [DOI] [PubMed] [Google Scholar]
- 8. Dalimi A, Abdoli A. Latent toxoplasmosis and human. Iran J Parasitool. 2012; 2 (1): 1– 17. [PMC free article] [PubMed] [Google Scholar]
- 9. Fekadu A, Shibre T, Cleare AJ. Toxoplasmosis as a cause for behavior disorders–overview of evidence and mechanisms. Folia Parasitol (Praha). 2010; 57 2: 105– 113. [DOI] [PubMed] [Google Scholar]
- 10. Miman O, Mutlu EA, Ozcan O, Atambay M, Karlidag R, Unal S. Is there any role of Toxoplasma gondii in the etiology of obsessive–compulsive disorder? Psychiatry Res. 2010; 177: 263– 265. [DOI] [PubMed] [Google Scholar]
- 11. Torrey EF, Bartko JJ, Yolken RH. Toxoplasma gondii and other risk factors for schizophrenia: an update. Schizophr Bull 2012; 38 3: 642– 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alipour A, Shojaee S, Mohebali M, Tehranidoost M, Abdi Masoleh F, Keshavarz H. Toxoplasma infection in schizophrenia patients: a comparative study withcontrol group. Iran J Parasitol. 2011; 6 (2): 31– 7. [PMC free article] [PubMed] [Google Scholar]
- 13. Blockland A. Acetylcholine: a neurotransmitter for learning and memory? Brain Res Brain Res Rev. 1995; 21: 285– 300. [DOI] [PubMed] [Google Scholar]
- 14. Tonin AA, Da Silva AS, Thomé GR, Sangoi MB, Oliveira LS, Flores MM, et al. Influence of toxoplasmosis on acetylcholinesterase activity, nitric oxide levels and cellularlesion on the brain of mice. Pathol Res Pract. 2014; 210 (8): 526– 32. [DOI] [PubMed] [Google Scholar]
- 15. Tonin AA, da Silva AS, Thorstenberg ML, Castilhos LG, França RT, Leal DB, et al. Influence of Toxoplasma gondii acute infection on cholinesterase activities of Wistar rats. Korean J Parasitol. 2013; 51 (4): 421– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu TS, Kim A, Kernie SG. Donepezil rescues spatial learning and memory deficits following traumatic brain injury independent of its effects on neurogenesis. PLoS One. 2015. 25; 10 (2): e0118793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saraei M, Ghaderi Y, Mosavi T, Shahnazi M, Keshavarz H, Shojaee S. Brain cystogenesis capacity of Toxoplasma gondii, avirulent Tehran strain in mice. Asian Pacific J Trop Dis. 2014; 4 (2): 739– 742. [Google Scholar]
- 18. Zhu Ch, Cui L, Zhang L. Comparison of a commercial ELISA with the modified agglutination test for detection of Toxoplasma gondii antibodies in sera of naturally infected dogs and cats. Iran J Parasitol. 2012; 7 (3): 89– 95. [PMC free article] [PubMed] [Google Scholar]
- 19. Aghaei I, Shabani I, Doustar N, Nazeri M, Dehpour A. Peroxisome proliferator-activated receptor-γ activation attenuates motor and cognition impairments induced by bile duct ligation in a rat model of hepatic cirrhosis. Pharmacol Biochem Behav. 2014; 120: 133– 139. [DOI] [PubMed] [Google Scholar]
- 20. Mahmoudvand H, Ziaali N, Aghaei I, Sheibani V, Shojaee S, Keshavarz H, Shabani M. The possible association between Toxoplasma gondii infection and risk of anxiety and cognitive disorders in BALB/c mice. Pathogen Glob Health. 2015, 109( 8), 369– 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mahmoudvand H, Saedi Dezaki E, Soleimani S, Baneshi MR, Kheirandish F, Ezatpour B, Zia-ali N. Seroprevalence and risk factors of Toxoplasma gondii infection among healthy blood donors in southeast of Iran. Parasite Immunol. 2015; 37 (7): 362– 367. [DOI] [PubMed] [Google Scholar]
- 22. Mahmoudvand H, Ziaali N, Ghazvini H, Shojaee S, Keshavarz H, Esmaeeilpour K, Sheibani V. Toxoplasma gondii Infection Promotes Neuroinflammation Through Cytokine Networks and Induced Hyperalgesia in BALB/c Mice. Inflammation. 2016, 39( 1), 405– 412. [DOI] [PubMed] [Google Scholar]
- 23. Zhou YH, Wang XB, Jiang SF, Xu YL, Tao JP, Zhang XP, et al. Impairment of learning and memory ability in mice with latent infection of Toxoplasma gondii. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2011; 29 (5): 333– 338. [PubMed] [Google Scholar]
- 24. Daniels BP, Sestito SR, Rouse ST. An expanded task battery in the Morris water maze reveals effects of Toxoplasmagondii infection on learning and memory in rats. Parasitol Int. 2015; 64 (1), 5–12. [DOI] [PubMed] [Google Scholar]
- 25. Jung BK, Pyo KH, Shin KY, Hwang YS, Lim H, Lee SJ, et al. Toxoplasma gondii infection in the brain inhibits neuronal degeneration and learning and memoryimpairments in a murine model of Alzheimer’s disease. PLoS One. 2012; 7 (3): e33312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haroon F, Handel U, Angenstein F, Goldschmidt J, Kreutzmann P, Lison H, et al. Toxoplasma gondii actively inhibits neuronal function in chronically infected mice. PLoS One. 2012; 7: e35516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Worth AR, Lymbery AJ, Thompson RC. Adaptive host manipulation by Toxoplasm gondii: fact or fiction? Trends Parasitol. 2013; 29: 150– 155. [DOI] [PubMed] [Google Scholar]
- 28. Novotná M, Hanusova J, Klose J, Preiss M, Havlicek J, Roubalová K, et al. Probable neuroimmunological link between Toxoplasma and cytomegalovirus infections and personality changes in the human host. BMC Infect Dis. 2005; 5: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Webster JP. The effect of Toxoplasma gondii on animal behavior: playing cat and mouse. Schizophr Bull. 2007; 33 3: 752– 756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Giacobini E. New trends in cholinergic therapy for Alzheimer’s disease: nicotin agonists or cholinesterases inhibitors? Prog Brain Res. 1996; 109: 311– 323. [DOI] [PubMed] [Google Scholar]
- 31. Easton A, Sankaranarayanan S, Tanghe A, Terwel D, Lin AX, Hoque N, Bourin C, Gu H, Ahlijanian M, Bristow L. Effects of sub-chronic donepezil on brain Abeta and cognition in a mouse model of Alzheimer’s disease. Psychopharmacology (Berl). 2013; 230 (2): 279– 289. [DOI] [PubMed] [Google Scholar]
- 32. Min D, Mao X, Wu K, Cao Y, Guo F, Zhu S, et al. Donepezil attenuates hippocampal neuronal damage and cognitive deficits after global cerebral ischemia in gerbils. Neurosci Lett. 2012; 510 (1): 29– 33. [DOI] [PubMed] [Google Scholar]