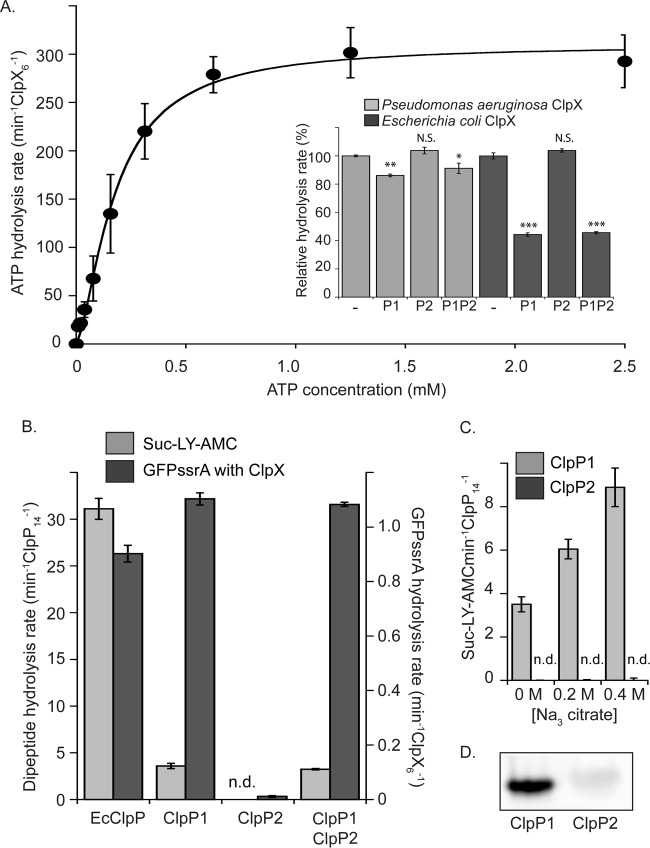
FIG 6.
Enzymatic activity of purified ClpX, ClpP1, and ClpP2. (A) The rate of ATP hydrolysis by P. aeruginosa ClpX increases as a function of ATP concentration. Data were fit to the Hill version of the Michaelis-Menten equation (line). Inset, ATP hydrolysis of ClpX from P. aeruginosa and E. coli is repressed by ClpP1 but not by ClpP2. Mixtures of ClpP1 and ClpP2 sat overnight at room temperature before use. Values are normalized averages (n = 3) ± SEM. Data were compared to control values (WT) by ANOVA with Dunnett's post hoc test (***, P < 0.0001; **, P < 0.01; *, P < 0.05; N.S., not significant). P. aeruginosa ClpX hydrolyzed ATP at a rate of 274.9 ± 1.2 min−1 · ClpX6−1, and E. coli ClpX hydrolyzed ATP at a rate of 104.1 ± 2.2 min−1 · ClpX6−1. (B) ClpP1 is enzymatically active, whereas ClpP2 is not. Degradation of small fluorogenic dipeptide substrate is shown with light gray bars, and degradation of degron-tagged GFP in conjunction with ClpX is shown with dark gray bars (n.d., not detected). The reaction rates measured for E. coli enzymes are shown for comparison. (C) Increasing sodium citrate concentration increases the peptidase activity of ClpP1 but has no effect on ClpP2. (D) ActivX TAMRA-FP serine hydrolase probe labels ClpP1 but not ClpP2. For all assays, values with error bars are averages (n = 3) ± SEM.