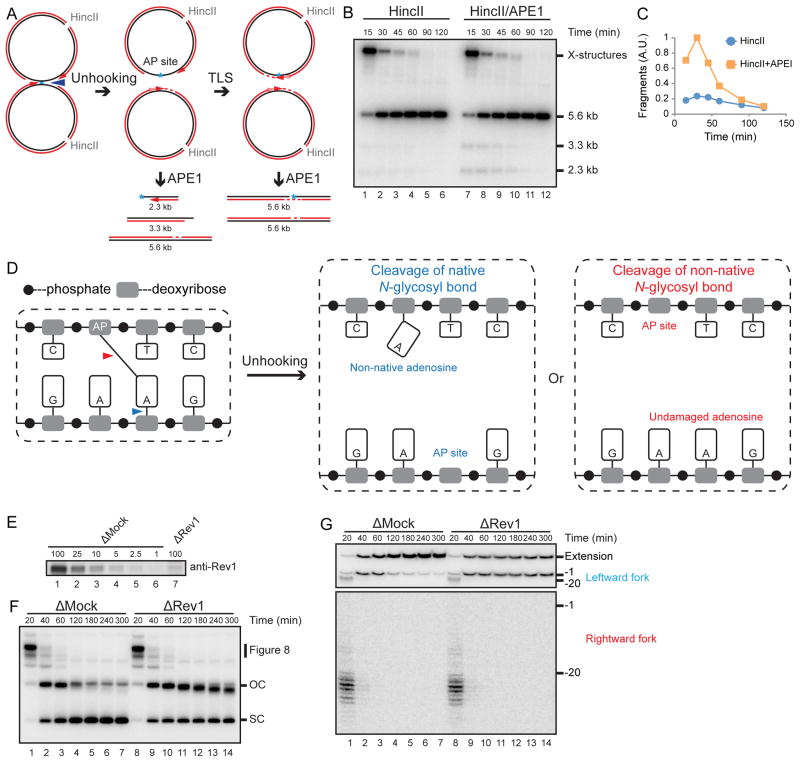
Figure 6. An AP-ICL is unhooked via cleavage of the non-native N-glycosyl bond.
(A) Expected species after digestion of pICLAP with HincII and APE1, including 3.3 kb and 2.3 kb fragments.
(B) pICLAP was replicated in egg extracts and then digested with HincII and APE1 as in Figure 2H.
(C) The 3.3 kb and 2.3 kb fragments in (B) were quantified and plotted.
(D) Two possible incision-independent unhooking pathways for an AP-ICL. Cleavage of the native N-glycosyl bond (blue arrowhead) yields a non-native adenosine and an AP site (middle panel), whereas cleavage of the non-native N-glycosyl bond (red arrowhead) yields an AP site and a normal adenosine (right panel).
(E) Mock-depleted and Rev1-depleted NPE were analyzed by Rev1 Western blotting. A relative volume of 100 corresponds to 0.66 μl NPE.
(F) pICLAP was replicated in mock- or Rev1-depleted egg extract in the presence of [α-32P]dATP and NMS-873 and analyzed as in Figure 1C.
(G) pICLAP was replicated in mock or Rev1 depleted egg extract in the presence of [α-32P]dATP and NMS-873. Samples were purified and digested with AflIII before separation on a denaturing polyacrylamide gel. Two portions of the autoradiograph are displayed with different contrast for optimal display.