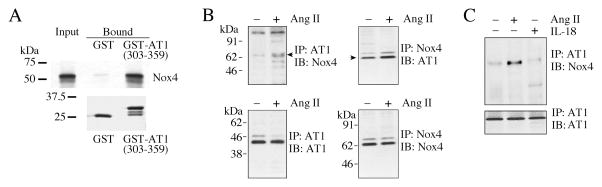
Fig. 6. Ang-II increases the physical association of AT1 and Nox4 in vitro and in vivo.
A, Nox4 binds the C-terminal domain of AT1 in vitro. A pull-down assay was carried out between a protein composed of the C-terminal cytoplasmic domain of AT1 fused to GST (GST-AT1(303-359)), pre-bound to GSH-sepharose beads, and in vitro transcribed/translated, 35S-methionine labeled mouse Nox4 ([35S]Met-Nox4). GST was used as a binding specificity control. Upper panel, aliquots of labeled Nox4 added to GST or GST-AT1(303-359) (equivalent to 2% of the total, “Input”), and the bound Nox4 eluted with SDS-PAGE treatment buffer from the GSH-sepharose beads after washing. Eluates (equivalent to 10% of eluate, “Bound”) were analyzed by SDS-PAGE and fluorography. Lower panel, to show the equal loading of the GSH-sepharose beads with GST and GST-AT1(303-359), equal volumes of the eluates were analyzed by SDS-PAGE and stained for protein with GelCode™ Blue Stain Reagent. B, AT1 and Nox4 association in vivo is increased by Ang-II. The quiescent CF were treated with or without Ang-II (10−7 M for 15 min), and AT1 and Nox4 binding was analyzed by co-immunoprecipitation/immunoblotting (co-IP/IB) using cleared cell lysates (left and right). All blots are representatives from three independent experiments. C, IL-18 fails to modulate AT1 binding to Nox4. The quiescent CF were treated with IL-18 (1 ng/ml for 15 min). Ang-II served as a positive control. Cell lysates were analyzed for AT1/Nox4 binding by co-IP/IB (left and right; n = 3).