Abstract
Objective
We aimed to investigate the association of diffuse myocardial fibrosis by cardiac magnetic resonance (CMR) T1 with complex ventricular arrhythmia (ComVA) in mitral valve prolapse (MVP).
Methods
A retrospective analysis was performed on 41 consecutive patients with MVP referred for CMR between 2006 and 2011, and 31 healthy controls. Arrhythmia analysis was available in 23 patients with MVP with Holter/event monitors. Left ventricular (LV) septal T1 times were derived from Look-Locker sequences after administration of 0.2 mmol/kg gadopentetate dimeglumine. Late gadolinium enhancement (LGE) CMR images were available for all subjects.
Results
Patients with MVP had significantly shorter postcontrast T1 times when compared with controls (334±52 vs 363±58 ms; p=0.03) despite similar LV ejection fraction (LVEF) (63±7 vs 60±6%, p=0.10). In a multivariable analysis, LV end-diastolic volume, LVEF and mitral regurgitation fraction were all correlates of T1 times, with LVEF and LV end-diastolic volume being the strongest (p=0.005, p=0.008 and p=0.045, respectively; model adjusted R2=0.30). Patients with MVP with ComVA had significantly shorter postcontrast T1 times when compared with patients with MVP without ComVA (324 (296, 348) vs 354 (327, 376) ms; p=0.03) and only 5/14 (36%) had evidence of papillary muscle LGE.
Conclusions
MVP may be associated with diffuse LV myocardial fibrosis as suggested by reduced postcontrast T1 times. Diffuse interstitial derangement is linked to subclinical systolic dysfunction, and may contribute to ComVA in MVP-related mitral regurgitation, even in the absence of focal fibrosis.
INTRODUCTION
Mitral valve prolapse (MVP) is a common valvulopathy affecting 2%–3% of the general population.1,2 MVP has been associated with an increased risk of arrhythmic complications including atrial/ventricular arrhythmias and sudden cardiac death (SCD).3–6 The prevalence of SCD in MVP has ranged between 1% and 2.5%.4,6,7 SCD has been reported to be more prevalent among patients with MVP with chordal rupture, flail mitral leaflet and severe mitral regurgitation (MR), suggesting that ventricular volume load may be an important trigger of ventricular arrhythmias. Recently, focal fibrosis was identified in the papillary muscles or inferolateral base on cardiac magnetic resonance (CMR) images with late gadolinium enhancement (LGE) in patients with MVP and complex ventricular arrhythmia (ComVA), SCD or both.8,9 Focal fibrosis was present even with only mild MR9 and was thought to be secondary to mechanical tethering of the myocardium by the prolapsing leaflets. Furthermore, increased frequency of complex ventricular ectopic activity has been shown to be associated with a predisposing phenotype of bileaflet MVP with an increased risk for out-of-hospital cardiac arrest.3 Clinical observations suggest that cases of SCD and MVP do not always fall into the categories of severe MR/flail leaflet or bileaflet MVP with mild regurgitation and focal fibrosis. In addition, the mechanism underlying ventricular arrhythmia associated with chronic left ventricular (LV) volume overload in longstanding severe primary MR is unclear.
Diffuse rather than focal myocardial fibrosis has been reported in MVP-related MR,10 but its association with ComVA in MVP is yet to be investigated. Myocardial tissue characterisation using T1 mapping allows for accurate quantitative assessment of diffuse myocardial fibrosis11 and has been validated by histological studies.12 Characterisation of the native T1 of myocardial tissue may be used to detect and assess various cardiomyopathies with prognostic significance.13,14 The aim of this study was to determine myocardial T1 in patients with MVP to assess for the presence of diffuse myocardial fibrosis and its association with ComVA.
METHODS
Study population
A query of our institutional electronic CMR database identified 44 patients with MVP referred for clinical CMR from 2006 to 2011. Three studies with poor LGE CMR images were excluded. None of the patients with MVP had coronary artery disease, hypertension or other intrinsic cardiomyopathies. Our control group consisted of 31 consecutively enrolled healthy volunteers free of significant cardiac disease based on clinical and CMR findings. The following baseline clinical and CMR variables were extracted from the electronic clinical database and CMR reports: age, gender, body surface area, systolic/diastolic blood pressure, New York Heart Association functional class, medication use, history of diabetes or tobacco use, estimated glomerular filtration rate (eGFR), contrast dose and time of sequence acquisition. The study was approved by the Beth Israel Deaconess Medical Center Institutional Review Board.
CMR protocol
CMR images were acquired using an Achieva 1.5 T MR whole body scanner (Philips Medical Systems, Best, the Netherlands) equipped with a 32-channel phased array coil. Breath-hold retrospectively ECG-gated cine steady state free processing (SSFP) images were acquired in the 2-chamber (2C) and 4-chamber (4C) horizontal long-axis views, and a short-axis stack covering the entire LV (8 mm slices with 2 mm gaps) as previously described.15,16 The LV outflow tract long-axis view was obtained by prescribing an image plane perpendicular to the mitral annular major axis centred at the aortic outflow track. In patients with MVP, a contiguous stack of cine SSFP images with 7 mm slice thickness (gap=0 mm) in the outflow tract view were obtained to cover the entire mitral valve.8,17 Free-breathing, ECG-triggered phase-contrast velocity sequences for ascending aortic flow oriented in the axial plane at the level of the bifurcation of the pulmonary artery were acquired as previously described.18 Fifteen minutes after administration of 0.2 mmol/kg gadopentetate dimeglumine (Magnevist, Berlex/Schering AG, Berlin, Germany), ECG-gated breath-hold two-dimensional (2D) LGE-CMR was performed in the 2C, 4C and short-axis orientations corresponding to the SSFP cine slices. Myocardial T1 times were calculated from a breath-hold Look-Locker sequence performed 15–25 min after the injection, with the following imaging parameters: repetition time (TR) 40 ms; echo time (TE) 5.9 ms; flip angle: 15°; 144×144 matrix; field of view (FOV) 400×400 mm; slice thickness 10 mm; echoplanar imaging (EPI) factor: 9. At 20 min after injection, an ECG-gated free breathing, respiratory navigator-gated three-dimensional (3D) LGE-CMR scan was obtained.8 Scan parameters for LGE acquisitions have been previously published.8 For both 2D and 3D LGE-CMR scans, fat saturation was applied and inversion times were determined using the Look-Locker sequence.
Image analysis
CMR data were analysed using commercially available software (ViewForum R5.1, Philips Medical Systems, Best, the Netherlands). MVP was defined as >2 mm displacement of the mitral leaflets into the left atrium as viewed in the LV outflow tract orientation (figure 1A).8,17 LV volumes were measured by tracing the end-diastolic and end-systolic LV endocardial contours in each short-axis slice and applying a summation of discs method. The LV ejection fraction (LVEF) was calculated as: 100×(LV end-diastolic volume−LV end-systolic volume)/LV end-diastolic volume. Effective LVEF was calculated as 100×(forward aortic stroke volume/LV end-diastolic volume). LV mass was measured by tracing end-diastolic endocardial and epicardial contours. The papillary muscles were considered part of the ventricular volumes. LV end-diastolic and end-systolic volumes and LV mass were indexed to body size (LVEDVI, LVESVI, LVMI). The MR volume was calculated as the difference between LV stroke volume and the forward aortic flow volume. The MR fraction (MRF) was obtained by dividing MR volume by the LV stroke volume. MR categories were graded as: 0 (none to trace) (<5%); 1+ (mild) (<16%); 2+ (moderate) (16%–25%); 3+ (moderate to severe) (26%–48%) and 4+ (severe) (>48%).18
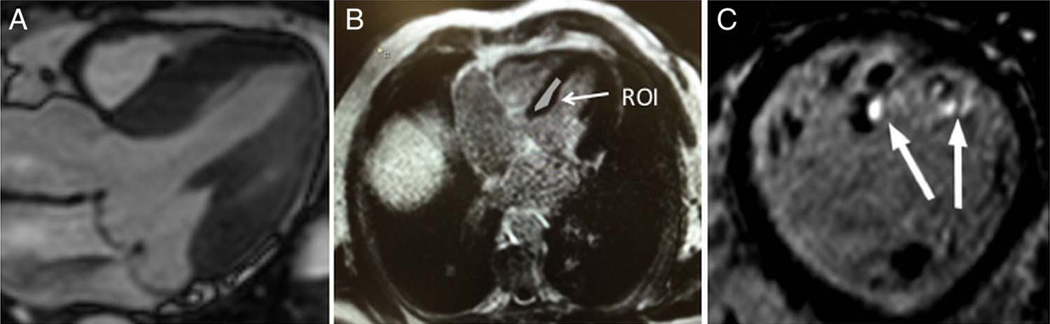
Figure 1.
(A) Cardiac magnetic resonance steady state free processing long-axis view of bileaflet mitral valve prolapse with central jet of mitral regurgitation. (B) Region of interest (arrow) manually drawn onto the mid-myocardial septum to measure T1 time. (C) Short-axis view with three-dimensional late-gadolinium enhancement showing fibrosis of the papillary muscle tips (arrows).
Myocardial T1 times were calculated from Look-Locker sequences with a custom built semi-automated tool (Matlab R2009b, Mathworks, Natick, Massachusetts, USA). The region of interest (ROI) was manually delineated in each T1-weighted image of the Look-Locker sequence at the mid-ventricular septum (figure 1B). Apparent T1 time was estimated by fitting the average T1-weighted signal (over each ROI) with a standard 3-parameter fit model of the T1 recovery signal.19 T1 time was finally estimated by correction of apparent T1 time using the ‘Look-Locker correction’ approach.19 The presence of myocardial LGE was determined when part of the myocardial tissue present on the SSFP images was replaced by high signal intensity in the LGE images (either 3D LGE alone or both 2D and 3D LGE) (figure 1C). As there was no evidence of septal LGE (see the Results section), none of the study subjects was excluded from T1 measurements because of the concomitant presence of LGE.
Arrhythmia analysis
Holter or event monitor arrhythmia data were available within 6 months of CMR in a subset of patients with MVP (n=23). ComVA was defined as grade III or higher by the Lown and Wolf classification.20 The absence of ComVA was determined by a negative Holter or event monitor, or by the absence of complaints of palpitations or skipped beats and no ambulatory ECG record of ventricular arrhythmias while being regularly followed by local cardiologists.
Statistical analysis
Clinical characteristics of the participants are presented as mean±SD or median with IQR for normally distributed and skewed continuous variables, respectively. The Shapiro-Wilk test was used to assess for normality. Comparisons between groups were made using Student’s t-test or Wilcoxon rank sum test for continuous data and χ2 or Fisher’s exact test for categorical data. The univariable association of T1 time with continuous variables (age, body surface area, systolic and diastolic blood pressure, LVEF, effective LVEF, LVEDVI, LVESVI, LVMI, MRF) was assessed using linear regression. T1 values by gender and those with or without MVP were assessed using the Wilcoxon rank sum test. Multivariable linear regression was used to determine the independent association of those variables with a statistically significant univariable association with T1 time. Since LVEDVI and LVESVI were highly correlated with each other, LVESVI was removed from the model to avoid multicollinearity. Covariates with a univariable p value of <0.1 (LVEF, LVEDVI, LVMI, MRF) were eligible for inclusion in the model. Age and gender were automatically included. We then assessed the univariable association of ComVA with age, gender, LVEF, LVEDVI, LVMI, T1 time, MRF and presence of LGE using logistic regression. Only T1 time had a univariable p value <0.1. Hence, multivariable logistic regression was not performed. A two-tailed p value of <0.05 was considered to be significant. All statistical analyses were performed using SAS (V.9.3 Statistical Analysis System, SAS Institute, Cary, North Carolina, USA).
RESULTS
Baseline clinical and CMR characteristics
A total of 72 subjects were included in the analysis: 41 MVP cases and 31 healthy controls. Among the cases, 21 (51%) had bileaflet MVP and 20 had unileaflet involvement (19 posterior and 1 anterior). Patients with MVP and controls were comparable with regards to most clinical characteristics except for a higher number of subjects with dyspnoea and medication use (table 1) among the MVP cases. LVESVI, LVMI and LVEF were also similar in the two groups (table 2). Patients with MVP had significantly lower effective LVEF (p<0.0001), higher LVEDVI (p=0.001) and greater MRF (p<0.0001) when compared with controls. The majority of patients with MVP (31/41 or 76%) had ≥2+ MR. Of these, five had a flail mitral leaflet (4/5 with isolated posterior MVP). Among the 21 patients with bileaflet MVP, 19/21 (90%) had ≥2+ MR, with one having a flail leaflet. Two patients with MVP had atrial fibrillation. Hence, MRF could not be calculated for these two individuals.
Table 1.
Demographic and clinical characteristics according to mitral valve prolapse status
| Mitral valve prolapse (n=41) |
Controls (n=31) |
p Value | |
|---|---|---|---|
| Age, years median (IQR) | 50 (47, 57) | 49 (37, 60) | 0.37 |
| Male, n (%) | 25 (61) | 18 (59) | 0.19 |
| BSA, m2 median (IQR) | 1.9 (1.72, 2.0) | 2.0 (1.8, 2.2) | 0.13 |
| SBP, mm Hg mean (SD) | 142 (26) | 146 (25) | 0.49 |
| DBP, mm Hg mean (SD) | 63 (11) | 62 (11) | 0.55 |
| Heart rate, beats/min median (IQR) | 63 (56,72) | 57 (51,67) | 0.14 |
| eGFR, mL/min/1.73 m2 mean (SD) | 70 (11) | 74 (10) | 0.06 |
| Dyspnoea, n (%) | 10 (24) | 0 (0) | <0.001 |
| NYHA class I, n (%) | 31 (76) | 31 (100) | 0.004 |
| NYHA class II, n (%) | 7 (17) | 0 (0) | 0.02 |
| NYHA class III, n (%) | 3 (7) | 0 (0) | 0.25 |
| NYHA class IV, n (%) | 0 (0) | 0 (0) | N/A |
| Current medication: | |||
| β-Blocker, n (%) | 15 (37) | 0 (0) | <0.001 |
| Calcium channel blocker, n (%) | 2 (5) | 0 (0) | 0.21 |
| ACE inhibitor, n (%) | 15 (37) | 0 (0) | <0.001 |
| ARB, n (%) | 0 (0) | 0 (0) | N/A |
| Diuretics, n (%) | 5 (12) | 0 (0) | 0.04 |
| Diabetes, n (%) | 2 (5) | 0 (0) | 0.21 |
| Current tobacco use, n (%) | 2 (5) | 0 (0) | 0.21 |
Categorical variables are presented as number of patients (%). Continuous variables are presented as median (IQR) or mean (SD).
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; BSA, body surface area; eGFR, estimated glomerular filtration rate; N/A, not applicable; NYHA, New York Heart Association functional class; SBP/DBP, systolic/diastolic blood pressure.
P-value <0.05 are shown in bold.
Table 2.
Cardiac magnetic resonance (CMR) characteristics according to mitral valve rolapsed status
| Mitral valve prolapse (n=41) |
Controls (n=31) |
p Value | |
|---|---|---|---|
| LVEDVI, mL/m2 | 103 (25) | 84 (17) | 0.001 |
| LVESVI, mL/m2 | 38 (13) | 33 (8) | 0.10 |
| LVMI, g/m2 | 59 (16) | 53 (13) | 0.10 |
| LVEF,% | 63 (7) | 60 (6) | 0.10 |
| Effective LVEF, % | 45 (9) | 55 (6) | <0.0001 |
| MRF,% | 28 (14) | 8 (6) | <0.0001 |
| LGE, n (%) | 11 (28) | 0 (0) | <0.0001 |
| Myocardial T1 time, ms | 334 (52) | 363 (58) | 0.03 |
| Gadopentetate dimeglumine, mmol/kg | 0.2 (0.03) | 0.2 (0.03) | 0.97 |
| T1 postcontrast acquisition time, min | 21 (4) | 21 (3) | 0.80 |
The categorical variable LGE is presented as number of patients with LGE (%). Remaining variables (all continuous) are presented as mean (SD).
LGE, late gadolinium enhancement; LVEDVI, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LVESVI, left ventricular end-systolic volume index; LVMI, left ventricular mass index; MRF, mitral regurgitation fraction.
P-value <0.05 are shown in bold.
Diffuse and focal fibrosis
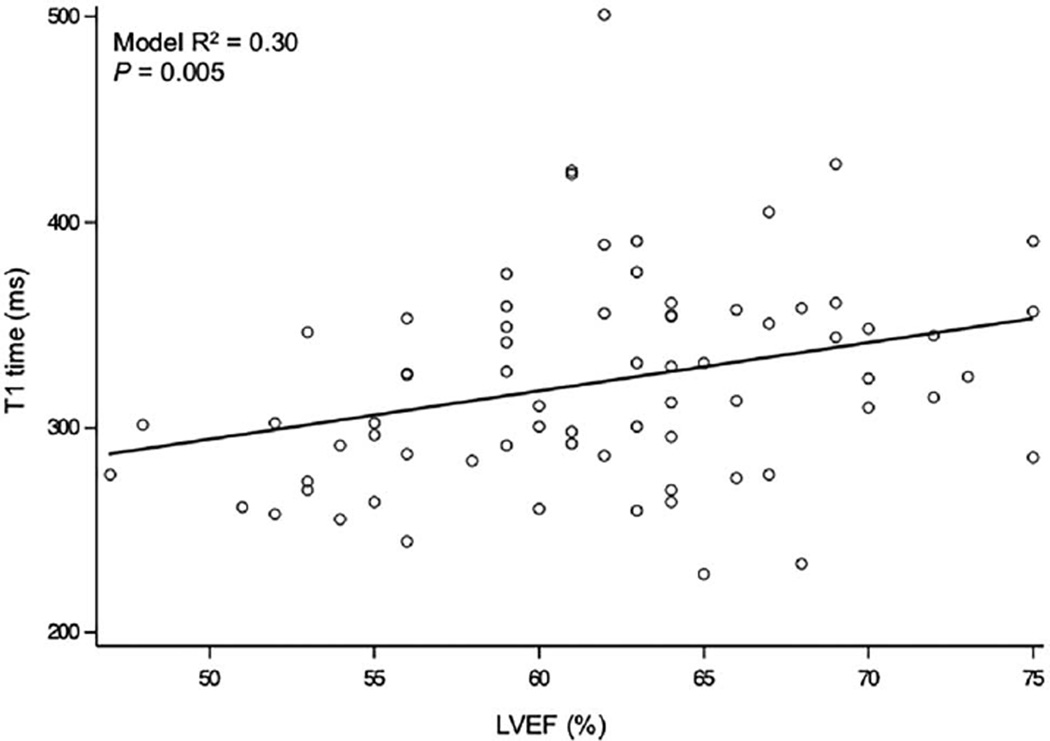
Overall, patients with MVP had significantly shorter postcontrast T1 times when compared with controls (p=0.03) (table 2 and figure 2). In the multivariable linear regression analysis (R2=0.30, adjusted R2=0.22), LVEF was an independent predictor of T1 times (β, 2.77; 95% CI 0.86 to 4.86; p=0.005) (table 3 and figure 3). LVEDVI was also strongly correlated with T1 (β, 1.10; 95% CI 0.30 to 1.89; p=0.008), whereas the association of MRF with shorter T1 times was borderline significant (β, −1.19; 95% CI −2.35 to −0.03; p=0.045) (table 3).
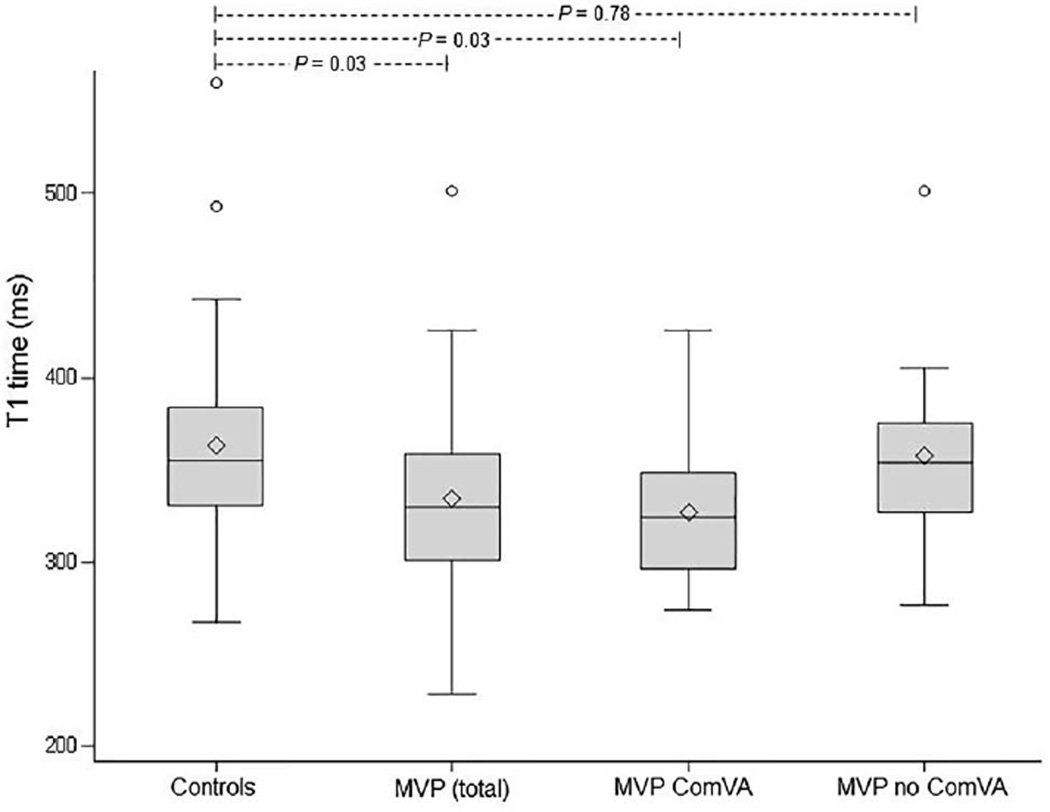
Figure 2.
Myocardial T1 times in patients with mitral valve prolapse (MVP) (total cohort, n=41), MVP with complex ventricular arrhythmia (ComVA, n=14) and MVP without ComVA (n=18) compared with controls (n=31).
Table 3.
Multivariable linear regression analysis to predict T1 times
| Variable | β | CI | p Value |
|---|---|---|---|
| MRF, per % | −1.19 | (−2.35 to −0.03) | 0.045 |
| LVEDVI, per mL/m2 | 1.10 | (0.30 to 1.89) | 0.008 |
| LVEF, per % | 2.77 | (0.86 to 4.68) | 0.005 |
| LVMI, per g/m2 | 0.32 | (−0.82 to 1.46) | 0.58 |
| Age, per year | 0.49 | (−0.33 to 1.32) | 0.24 |
| Male gender | 1.42 | (−25.29 to 28.12) | 0.916 |
The multivariable linear regression model was adjusted for the following covariates: MRF, LVEDVI, LVEF, LVMI, age and male gender.
LVEDVI, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; MRF, mitral regurgitation fraction.
P-value <0.05 are shown in bold.
Figure 3.
Simple bivariate scatter plot demonstrating the correlation between T1 times and left ventricular ejection fraction (LVEF).
LGE was found only in the MVP group (table 2) in 11/41 subjects. All 11 patients with MVP with LGE had evidence of papillary muscle fibrosis (figure 1C). In addition to papillary muscle involvement, one subject had mid-myocardial LGE of the basal inferolateral wall. None of the patients with MVP had LGE in the interventricular septum.
Ventricular arrhythmia
Among patients with MVP with available information on arrhythmia (n=32, of which 23 were monitor based), there were 14 individuals with ComVA and 18 without ComVA (9 based on negative Holter or event monitor and 9 based on the absence of complaints of palpitations or skipped beats and no ambulatory ECG record of ventricular arrhythmias). Clinical characteristics of the two groups are shown in table 4. Patients with MVP with ComVA had significantly shorter postcontrast T1 times when compared with patients with MVP without ComVA (p=0.03) despite similar demographics, LV volumes, LV mass, LVEF and MRF (table 4). Furthermore, patients with MVP with ComVA, but not patients with MVP without ComVA, had significantly shorter T1 times when compared with controls (p=0.03) (figure 2). Among the patients with MVP with ComVA, only 36% (5/14) had LGE (4 out of 5 with ≥3+ MR). This proportion was not significantly different in the group of patients with MVP without ComVA (LGE in 3/18 or 17%), Fisher’s p=0.25 (table 4). Shorter T1 time was the only predictor of ComVA in a univariable logistic regression analysis including age, gender, LVEF, LVEDVI, LVMI, MRF and LGE (OR 0.98; 95% CI 0.96 to 1.00; p=0.04; c-statistic 0.73).
Table 4.
Demographic and cardiac magnetic resonance (CMR) characteristics of mitral valve prolapse (MVP) patients according to complex ventricular arrhythmia (ComVA)
| ComVA (n=14) |
No ComVA (n=18) |
p Value | |
|---|---|---|---|
| Age, years | 55 (13) | 51 (10) | 0.27 |
| Male, n (%) | 10 (71) | 11 (61) | 0.71 |
| BSA, m2 | 1.9 (0.2) | 1.9 (0.2) | 0.95 |
| LVEDVI, mL/m2 | 114 (92 to 119) | 108 (91 to 120) | 0.88 |
| LVESVI, ml/m2 | 40 (17) | 40 (11) | 0.99 |
| LVMI, g/m2 | 64 (19) | 59 (14) | 0.20 |
| LVEF, % | 63 (8) | 61 (6) | 0.13 |
| Effective LVEF, % | 46 (10) | 41 (8) | 0.31 |
| MRF, % | 26 (15) | 32 (14) | 0.75 |
| LGE, n (%) | 5 (36) | 3 (17) | 0.25 |
| Myocardial T1 time, ms | 324 (296 to 348) | 354 (327 to 376) | 0.03 |
Values in parentheses are percentages for categorical and SDs or IQR for continuous variables.
BSA, body surface area; LGE, late gadolinium enhancement; LVEDVI, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LVESVI, left ventricular end-systolic volume index; LVMI, left ventricular mass index; MRF, mitral regurgitation fraction.
P-value <0.05 are shown in bold.
DISCUSSION
In this retrospective study of patients with MVP, we found that MVP was associated with reduced postcontrast T1 times suggestive of diffuse myocardial fibrosis. In addition, patients with MVP with ComVA had significantly shorter postcontrast T1 times when compared with patients with MVP without ComVA. Diffuse interstitial derangement was associated with subclinical systolic dysfunction and may represent the mechanism of ComVA in chronic MVP-related MR, even in the absence of focal fibrosis.
Using CMR, a prior investigation has demonstrated abnormal T1 and diffuse myocardial fibrosis in asymptomatic patients with moderate-to-severe MVP-related MR.10 Similarly, our patient population includes mostly MVP subjects with ≥2+ MR and our data also highlights the association between lower (although preserved) LVEF and abnormal T1. Nevertheless, our study is the first to demonstrate that diffuse interstitial fibrosis is independently associated with ComVA in MVP, even after adjusting for severity of MR, LV cavity size/function and presence of LGE. Only the MVP group with ComVA had a significantly shorter T1 when compared with controls. This finding further highlights the potential role of diffuse fibrosis in MVP-related arrhythmia.
Patients with MVP in our study sample had predominant bileaflet involvement, a phenotype previously correlated with arrhythmic risk in both clinical and pathological studies.3,9 In these investigations, the severity of MR was overall mild, and most patients had evidence of focal fibrosis in the inferolateral base or papillary muscles. In contrast, we found that focal fibrosis was present in a minority of patients with MVP and ComVA, and LGE was not the major predictor of arrhythmic risk. Traditionally, increased MR associated with a flail mitral leaflet or ruptured chordae has been reported to increase arrhythmic risk among patients with MVP,21–22 as abnormal autonomic tone resulting from LV volume overload may be a predisposing factor to lethal arrhythmia.23 In our cohort, only the minority of subjects with ≥3+ MR (5/31) had a flail leaflet. Therefore, diffuse myocardial fibrosis may be the substrate of ventricular arrhythmia in the context of significant, longstanding primary MR not driven by a flail leaflet, and outside the rare scenario of mild MR with focal fibrosis.
Whereas lower LVEF was an independent predictor of shorter postcontrast T1 times in our multivariable analysis, the association of MR with T1 was borderline significant (p=0.045). Although subclinical LV systolic dysfunction is most likely secondary to MR, the data are insufficient to completely exclude a primary myopathy among our study subjects. Interestingly, 2 of our 14 patients with ComVA had no or trivial MR, no papillary muscle LGE and T1 times ≤350. Recently, animal and in vitro studies have demonstrated that overexpression of transforming growth factor (TGF)-β contributes to fibrosis and matrix remodelling in MVP.24–25 Whereas focal fibrosis is thought to be secondary to local traction on the papillary muscles and inferolateral wall by the prolapsing leaflets, it is plausible that diffuse fibrosis is rather the consequence of high levels of a profibrotic cytokine, even in the absence of MR. Furthermore, histological studies have demonstrated diffuse myocardial fibrosis in a small subgroup of patients with SCD without clear structural heart disease.26 Expression of TGF-β was significantly increased compared with controls, suggesting the role of TGF-β as a potential mediator of interstitial remodelling in SCD.
In patients with MVP, premature ventricular contractions or ventricular tachycardia have been shown to arise from the fascicles, LV outflow tract or septal aspect of the mitral annulus on electrophysiological study.3 As no focal fibrosis has been described by imaging or pathological studies in these sites, it is plausible that the mechanism of arrhythmia differs from the one underlying focal scar in the papillary muscle or the basal inferolateral LV. We can postulate the existence of various mechanisms for ventricular arrhythmia in the MVP population. Focal myocardial fibrosis (positive LGE) with consequent regions of conduction block may promote re-entry-mediated arrhythmia. In addition, abnormal myocytes in the setting of subclinical myocardial dysfunction and associated interstitial fibrosis (abnormal T1) can manifest spontaneous diastolic depolarisation resulting in abnormal automaticity. Finally, diffuse myocardial fibrosis in the setting of chronic MR, volume loading and LV dilatation could promote enhanced sympathetic tone and triggered-mediated sustained ventricular arrhythmias. Further studies are needed to elucidate the pathophysiology of MVP-related ventricular arrhythmia.
Limitations
Our study has several limitations. First, our sample size is modest, and even smaller when further stratifying by presence or absence of ComVA. Some of the statistically non-significant comparisons may be related to limited power to discern differences. Not all potential predictors of T1 times could be included in the multivariable regression model due to the small sample size and overfitting. Second, given the retrospective nature of our study, the prevalence of SCD could not be fully ascertained in a longitudinal fashion. ComVA was used as surrogate of arrhythmic risk based on recent literature reporting that ComVA is associated with a predisposing phenotype of bileaflet MVP with an increased risk for out-of-hospital cardiac arrest.3 Indeed, the majority of our patients with MVP had traditional features of ‘malignant’ MVP, specifically bileaflet MVP with or without significant MR. Third, our CMR protocol did not include measures of native T1 or extracellular volume, as these methods were not published at the time of study inception. Although assessment of postcontrast T1 times is a histologically validated technique,27 we acknowledge that factors such as heart rate, eGFR and time of sequence acquisition may affect postcontrast T1 times. In our study, there were no significant differences between MVP subjects and controls with regards to these theoretical confounders (tables 1 and 2). Lastly, assessment of LGE was qualitative and not quantitative.
Clinical and research implications
Diffuse interstitial derangement may contribute to ComVA in MVP-related MR, even without the presence of focal fibrosis. Clinical follow-up with repeat CMR studies in a larger patient cohort may help clarify if diffuse myocardial fibrosis represents a precursor of focal fibrosis or an independent predictor of SCD. T1 mapping by CMR may become an additional marker of arrhythmic risk in MVP-related MR and also in early stages of MVP progression without significant MR.
CONCLUSIONS
MVP may be associated with reduced postcontrast T1 times suggestive of diffuse LV myocardial fibrosis. Diffuse interstitial derangement is associated with subclinical LV systolic dysfunction, and may represent the mechanism of ComVA in MVP-related MR, despite the absence of focal fibrosis. Prospective studies are needed to establish if diffuse myocardial fibrosis is a precursor of focal fibrosis and/or predictor of SCD in this population.
Key messages.
What is already known on this subject?
Mitral valve prolapse (MVP) is a common valvulopathy affecting 2–3% of the general population and has been associated with an increased risk of arrhythmic complications including atrial/ventricular arrhythmias and sudden cardiac death (SCD).
What might this study add?
MVP may be associated with reduced postcontrast T1 times suggestive of diffuse left ventricular (LV) myocardial fibrosis. Diffuse interstitial derangement is associated with subclinical LV systolic dysfunction, and may represent the mechanism of complex ventricular arrhythmias in MVP-related mitral regurgitation (MR), despite the absence of focal fibrosis.
How might this impact on clinical practice?
T1 mapping by cardiac magnetic resonance imaging (CMR) may become an additional marker of arrhythmic risk in MVP-related MR. Clinical follow-up with repeat CMR studies in a larger patient cohort may help clarify if diffuse myocardial fibrosis represents a precursor of focal fibrosis or an independent predictor of SCD.
Acknowledgments
Funding National Institutes of Health (NIH): National Heart, Lung, and Blood Institute. NIH K23HL116652 (FND).
Footnotes
Contributors FND conceived of the study. AHB and FND initiated the study design and AHB primarily drafted the manuscript. SR, MF, KVK, BG, PJZ, WJM, RN and FND helped with implementation. THH and LHN provided statistical expertise and conducted the primary statistical analysis. All authors contributed to refinement of the study protocol and approved the final manuscript.
Competing interests None declared.
Ethics approval Institutional Review Board.
REFERENCES
- 1.Freed LA, Levy D, Levine RA, et al. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med. 1999;341:1–7. doi: 10.1056/NEJM199907013410101. [DOI] [PubMed] [Google Scholar]
- 2.Freed LA, Benjamin EJ, Levy D, et al. Mitral valve prolapse in the general population: the benign nature of echocardiographic features in the Framingham Heart Study. J Am Coll Cardiol. 2002;40:1298–1304. doi: 10.1016/s0735-1097(02)02161-7. [DOI] [PubMed] [Google Scholar]
- 3.Sriram CS, Syed FF, Ferguson ME, et al. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. J Am Coll Cardiol. 2013;62:222–230. doi: 10.1016/j.jacc.2013.02.060. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura RA, McGoon MD, Shub C, et al. Echocardiographically documented mitral-valve prolapse. Long-term follow-up of 237 patients. N Engl J Med. 1985;313:1305–1309. doi: 10.1056/NEJM198511213132101. [DOI] [PubMed] [Google Scholar]
- 5.Vohra J, Sathe S, Warren R, et al. Malignant ventricular arrhythmias in patients with mitral valve prolapse and mild mitral regurgitation. Pacing Clin Electrophysiol. 1993;16:387–393. doi: 10.1111/j.1540-8159.1993.tb01599.x. [DOI] [PubMed] [Google Scholar]
- 6.Düren DR, Becker AE, Dunning AJ. Long-term follow-up of idiopathic mitral valve prolapse in 300 patients: a prospective study. J Am Coll Cardiol. 1988;11:42–47. doi: 10.1016/0735-1097(88)90164-7. [DOI] [PubMed] [Google Scholar]
- 7.Narayanan K, Uy-Evanado A, Teodorescu C, et al. Mitral valve prolapse and sudden cardiac arrest in the community. Heart Rhythm. 2016;13:498–503. doi: 10.1016/j.hrthm.2015.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han Y, Peters DC, Salton CJ, et al. Cardiovascular magnetic resonance characterization of mitral valve prolapse. JACC Cardiovasc Imaging. 2008;1:294–303. doi: 10.1016/j.jcmg.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Basso C, Perazzolo Marra M, Rizzo S, et al. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation. 2015;132:556–566. doi: 10.1161/CIRCULATIONAHA.115.016291. [DOI] [PubMed] [Google Scholar]
- 10.Edwards NC, Moody WE, Yuan M, et al. Quantification of left ventricular interstitial fibrosis in asymptomatic chronic primary degenerative mitral regurgitation. Circ Cardiovasc Imaging. 2014;7:946–953. doi: 10.1161/CIRCIMAGING.114.002397. [DOI] [PubMed] [Google Scholar]
- 11.Jellis CL, Kwon DH. Myocardial T1 mapping: modalities and clinical applications. Cardiovasc Diagn Ther. 2014;4:126–137. doi: 10.3978/j.issn.2223-3652.2013.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Meester de Ravenstein C, Bouzin C, Lazam S, et al. Histological Validation of measurement of diffuse interstitial myocardial fibrosis by myocardial extravascular volume fraction from Modified Look-Locker imaging (MOLLI) T1 mapping at 3 T. J Cardiovasc Magn Reson. 2015;17:48. doi: 10.1186/s12968-015-0150-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moon JC, Messroghli DR, Kellman P, et al. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15:92. doi: 10.1186/1532-429X-15-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong TC, Piehler K, Meier CG, et al. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126:1206–1216. doi: 10.1161/CIRCULATIONAHA.111.089409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chuang ML, Hibberd MG, Salton CJ, et al. Importance of imaging method over imaging modality in noninvasive determination of left ventricular volumes and ejection fraction: assessment by two- and three-dimensional echocardiography and magnetic resonance imaging. J Am Coll Cardiol. 2000;35:477–484. doi: 10.1016/s0735-1097(99)00551-3. [DOI] [PubMed] [Google Scholar]
- 16.Salton CJ, Chuang ML, O’Donnell CJ, et al. Gender differences and normal left ventricular anatomy in an adult population free of hypertension. A cardiovascular magnetic resonance study of the Framingham Heart Study Offspring cohort. J Am Coll Cardiol. 2002;39:1055–1060. doi: 10.1016/s0735-1097(02)01712-6. [DOI] [PubMed] [Google Scholar]
- 17.Delling FN, Kang LL, Yeon SB, et al. CMR predictors of mitral regurgitation in mitral valve prolapse. JACC Cardiovasc Imaging. 2010;3:1037–1045. doi: 10.1016/j.jcmg.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Gelfand EV, Hughes S, Hauser TH, et al. Severity of mitral and aortic regurgitation as assessed by cardiovascular magnetic resonance: optimizing correlation with Doppler echocardiography. J Cardiovasc Magn Reson. 2006;8:503–507. doi: 10.1080/10976640600604856. [DOI] [PubMed] [Google Scholar]
- 19.Deichmann R, Haase A. Quantification of T1 values by SNAPSHOT-FLASH NMR imaging. J Magn Reson. 1992;96:608–612. [Google Scholar]
- 20.Lown B, Wolf M. Approaches to sudden death from coronary heart disease. Circulation. 1971;44:130–142. doi: 10.1161/01.cir.44.1.130. [DOI] [PubMed] [Google Scholar]
- 21.Grigioni F, Enriquez-Sarano M, Ling LH, et al. Sudden death in mitral regurgitation due to flail leaflet. J Am Coll Cardiol. 1999;34:2078–2085. doi: 10.1016/s0735-1097(99)00474-x. [DOI] [PubMed] [Google Scholar]
- 22.Davies MJ, Moore BP, Braimbridge MV. The floppy mitral valve. Study of incidence, pathology, and complications in surgical, necropsy, and forensic material. Br Heart J. 1978;40:468–481. doi: 10.1136/hrt.40.5.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein KM, Borer JS, Hochreiter C, et al. Prognostic value and physiological correlates of heart rate variability in chronic severe mitral regurgitation. Circulation. 1993;88:127–135. doi: 10.1161/01.cir.88.1.127. [DOI] [PubMed] [Google Scholar]
- 24.Hagler MA, Hadley TM, Zhang H, et al. TGF-β signalling and reactive oxygen species drive fibrosis and matrix remodelling in myxomatous mitral valves. Cardiovasc Res. 2013;99:175–184. doi: 10.1093/cvr/cvt083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geirsson A, Singh M, Ali R, et al. Modulation of transforming growth factor-β signaling and extracellular matrix production in myxomatous mitral valves by angiotensin II receptor blockers. Circulation. 2012;126(Suppl 1):S189–S197. doi: 10.1161/CIRCULATIONAHA.111.082610. [DOI] [PubMed] [Google Scholar]
- 26.John BT, Tamarappoo BK, Titus JL, et al. Global remodeling of the ventricular interstitium in idiopathic myocardial fibrosis and sudden cardiac death. Heart Rhythm. 2004;1:141–149. doi: 10.1016/j.hrthm.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 27.Iles L, Pfluger H, Phrommintikul A, et al. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol. 2008;52:1574–1580. doi: 10.1016/j.jacc.2008.06.049. [DOI] [PubMed] [Google Scholar]