Abstract
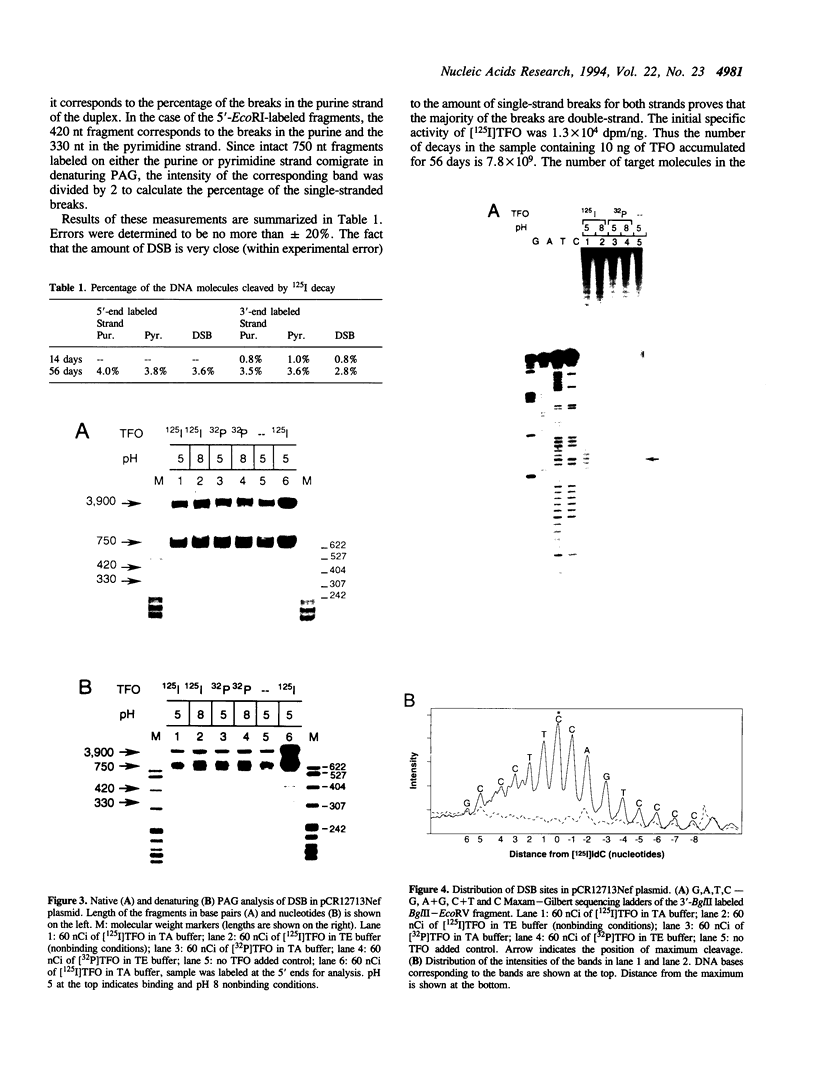
A triplex-forming oligonucleotide (TFO) complementary to the polypurine-polypyrimidine region of the nef gene of the Human Immunodeficiency Virus (HIV) was labeled with 125I at the C5 position of a single deoxycytosine residue. Labeled TFO was incubated with a plasmid containing a fragment of the nef gene. Decay of 125I was found to cause double-strand breaks (DSB) within the nef gene upon triplex formation in a sequence specific manner. No DSB were detected after incubation at ionic conditions preventing triplex formation or when TFO was labeled with 32P instead of 125I. Mapping DSB sites with single base resolution showed that they are distributed within 10 bp of a maximum located exactly opposite the position of the [125I] IdC in the TFO. We estimate that on average the amount of DSB produced per decay is close to one.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burki H. J., Roots R., Feinendegen L. E., Bond V. P. Inactivation of mammalian cells after disintegration of 3H or 125I in cell DNA at -196 degrees C. Int J Radiat Biol Relat Stud Phys Chem Med. 1973 Oct;24(4):363–375. doi: 10.1080/09553007314551221. [DOI] [PubMed] [Google Scholar]
- England P. R., Murray V. Sequence-specific DNA damage using iodine-125-labeled antisense oligonucleotides. Antisense Res Dev. 1993 Summer;3(2):219–224. doi: 10.1089/ard.1993.3.219. [DOI] [PubMed] [Google Scholar]
- Gibbs R. A., Camakaris J., Hodgson G. S., Martin R. F. Molecular characterization of 125I decay and X-ray-induced HPRT mutants in CHO cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1987 Feb;51(2):193–199. doi: 10.1080/09553008714550691. [DOI] [PubMed] [Google Scholar]
- Giovannangéli C., Rougée M., Garestier T., Thuong N. T., Hélène C. Triple-helix formation by oligonucleotides containing the three bases thymine, cytosine, and guanine. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8631–8635. doi: 10.1073/pnas.89.18.8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer K. G., Harris C. R., Smith J. M. Rdiotoxicity of intracellular 67Ga, 125I and 3H. Nuclear versus cytoplasmic radiation effects in murine L1210 leukaemia. Int J Radiat Biol Relat Stud Phys Chem Med. 1975 Sep;28(3):225–241. doi: 10.1080/09553007514550991. [DOI] [PubMed] [Google Scholar]
- Kassis A. I., Adelstein S. J., Haydock C., Sastry K. S., McElvany K. D., Welch M. J. Lethality of Auger electrons from the decay of bromine-77 in the DNA of mammalian cells. Radiat Res. 1982 May;90(2):362–373. [PubMed] [Google Scholar]
- Kassis A. I., Fayad F., Kinsey B. M., Sastry K. S., Adelstein S. J. Radiotoxicity of an 125I-labeled DNA intercalator in mammalian cells. Radiat Res. 1989 May;118(2):283–294. [PubMed] [Google Scholar]
- Kassis A. I., Fayad F., Kinsey B. M., Sastry K. S., Taube R. A., Adelstein S. J. Radiotoxicity of 125I in mammalian cells. Radiat Res. 1987 Aug;111(2):305–318. [PubMed] [Google Scholar]
- Kassis A. I., Sastry K. S., Adelstein S. J. Kinetics of uptake, retention, and radiotoxicity of 125IUdR in mammalian cells: implications of localized energy deposition by Auger processes. Radiat Res. 1987 Jan;109(1):78–89. [PubMed] [Google Scholar]
- Krisch R. E., Sauri C. J. Further studies of DNA damage and lethality from the decay of iodine-125 in bacteriophages. Int J Radiat Biol Relat Stud Phys Chem Med. 1975 Jun;27(6):553–560. doi: 10.1080/09553007514550581. [DOI] [PubMed] [Google Scholar]
- Lyamichev V. I., Mirkin S. M., Frank-Kamenetskii M. D., Cantor C. R. A stable complex between homopyrimidine oligomers and the homologous regions of duplex DNAs. Nucleic Acids Res. 1988 Mar 25;16(5):2165–2178. doi: 10.1093/nar/16.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makrigiorgos G. M., Berman R. M., Baranowska-Kortylewicz J., Bump E., Humm J. L., Adelstein S. J., Kassis A. I. DNA damage produced in V79 cells by DNA-incorporated iodine-123: a comparison with iodine-125. Radiat Res. 1992 Mar;129(3):309–314. [PubMed] [Google Scholar]
- Martin R. F., Haseltine W. A. Range of radiochemical damage to DNA with decay of iodine-125. Science. 1981 Aug 21;213(4510):896–898. doi: 10.1126/science.7256283. [DOI] [PubMed] [Google Scholar]
- Martin R. F., Holmes N. Use of an 125I-labelled DNA ligand to probe DNA structure. 1983 Mar 31-Apr 6Nature. 302(5907):452–454. doi: 10.1038/302452a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mirkin S. M., Frank-Kamenetskii M. D. H-DNA and related structures. Annu Rev Biophys Biomol Struct. 1994;23:541–576. doi: 10.1146/annurev.bb.23.060194.002545. [DOI] [PubMed] [Google Scholar]
- Miyazaki N., Shinohara K. Cell killing induced by decay of 125I during the cell cycle: comparison of 125I-antipyrine with 125I-bovine serum albumin. Radiat Res. 1993 Feb;133(2):182–186. [PubMed] [Google Scholar]
- Moser H. E., Dervan P. B. Sequence-specific cleavage of double helical DNA by triple helix formation. Science. 1987 Oct 30;238(4827):645–650. doi: 10.1126/science.3118463. [DOI] [PubMed] [Google Scholar]
- Murray V., Martin R. F. Sequence specificity of 125I-labelled Hoechst 33258 in intact human cells. J Mol Biol. 1988 May 20;201(2):437–442. doi: 10.1016/0022-2836(88)90150-7. [DOI] [PubMed] [Google Scholar]
- Panyutin I. G., Hsieh P. Formation of a single base mismatch impedes spontaneous DNA branch migration. J Mol Biol. 1993 Mar 20;230(2):413–424. doi: 10.1006/jmbi.1993.1159. [DOI] [PubMed] [Google Scholar]
- Sastry K. S. Biological effects of the Auger emitter iodine-125: a review. Report No. 1 of AAPM Nuclear Medicine Task Group No. 6. Med Phys. 1992 Nov-Dec;19(6):1361–1370. doi: 10.1118/1.596926. [DOI] [PubMed] [Google Scholar]
- Volkmann S., Dannull J., Moelling K. The polypurine tract, PPT, of HIV as target for antisense and triple-helix-forming oligonucleotides. Biochimie. 1993;75(1-2):71–78. doi: 10.1016/0300-9084(93)90027-p. [DOI] [PubMed] [Google Scholar]
- Voloshin O. N., Mirkin S. M., Lyamichev V. I., Belotserkovskii B. P., Frank-Kamenetskii M. D. Chemical probing of homopurine-homopyrimidine mirror repeats in supercoiled DNA. Nature. 1988 Jun 2;333(6172):475–476. doi: 10.1038/333475a0. [DOI] [PubMed] [Google Scholar]