Figure 1.
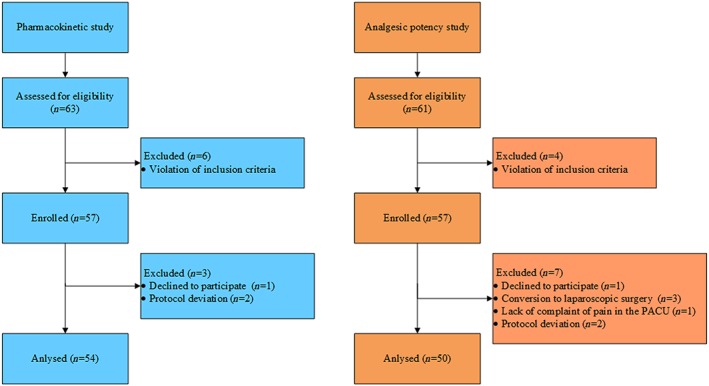
Consort diagram of participants in the two studies. For the pharmacokinetic study, a total of 63 patients were screened, and of these, six patients were excluded due to violations of the inclusion criteria. A total of 57 patients were enrolled in this study, and seven patients dropped out from the study because of withdrawal of consent before administration of oxycodone (n = 1) and protocol deviations (n = 2). Hence, 54 patients were included in the safety and pharmacokinetic analyses. For the analgesic potency study, 61 patients were screened, and of these, four patients were excluded due to violations of the inclusion criteria. A total of 57 patients were enrolled in this study, and five patients dropped out from the study because of conversion to laparoscopic surgery (n = 3), no complaint of pain at PACU (n = 1), and withdrawal of consent at PACU (n = 1). Additionally, two patients were excluded from the evaluation of MEC, MEAC and analgesic potency of oxycodone because of protocol deviations. Hence, 52 and 50 patients were included in the safety and the MEAC analyses, respectively. PACU: post‐anaesthesia care unit
