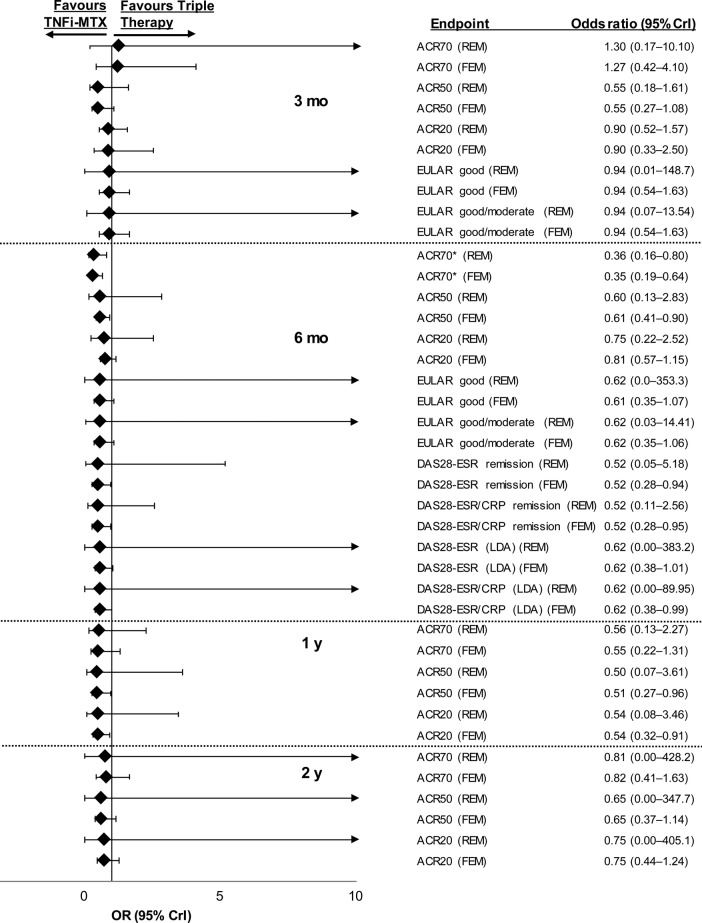
Figure 2.
Relative treatment effects concerning efficacy endpoints in patients with inadequate response to methotrexate for triple therapy versus TNFi–methotrexate at 3 months, 6 months, 1 year and 2 years. ACR70/50/20, American College of Rheumatology 70%/50%/20% response criteria; CrI, credible interval; CRP, C-reactive protein; DAS28, Disease Activity Score including 28-joint count; ESR, erythrocyte sedimentation rate; EULAR, European League Against Rheumatism; FEM, fixed-effects model; LDA, low disease activity; MTX, methotrexate; REM, random-effects model; TNFi, tumour necrosis factor inhibitor. *ACR70 at 6 months was a prespecified primary endpoint.