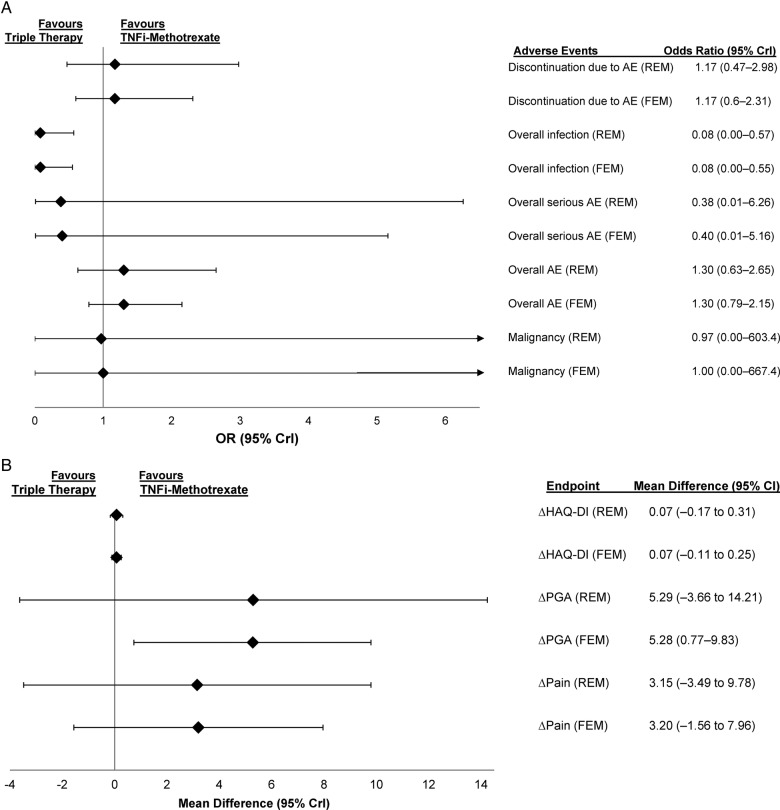
Figure 4.
(A) ORs comparing safety outcomes and (B) mean differences in patient-reported outcome endpoints in patients treated with triple therapy versus TNFi–methotrexate in patients who had inadequate response to methotrexate at 6 months. AE, adverse event; CrI, credible interval; FEM, fixed-effects model; HAQ-DI, Health Assessment Questionnaire-Disability Index; PGA, patient general assessment; REM, random-effects model; TNFi, tumour necrosis factor inhibitor.