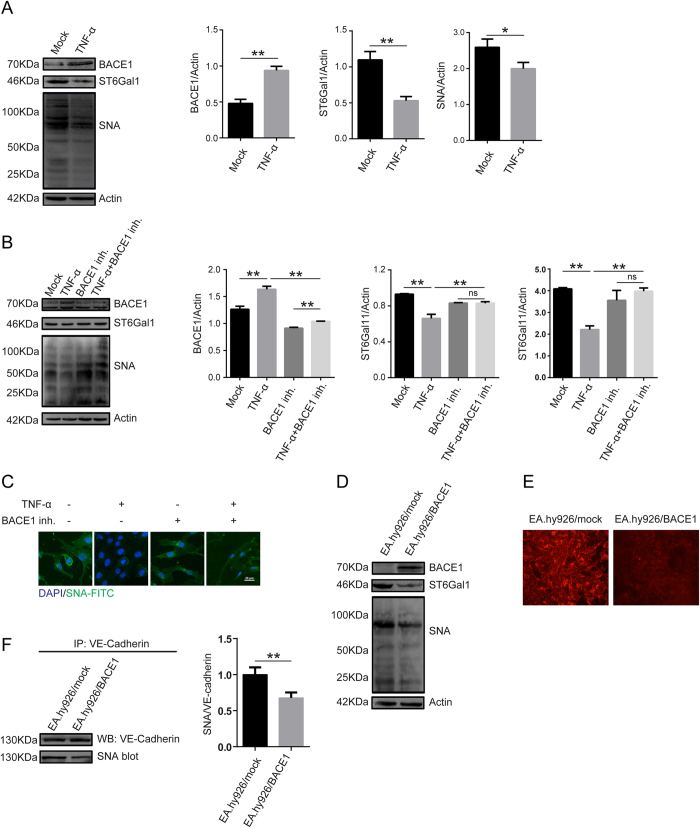
Figure 4. TNF-α regulates ST6Gal-I levels through BACE1.
(A) EA.hy926 cells were treated with TNF-α for 24 h, and then BACE1, ST6Gal-I and total protein α-2, 6 sialylation levels were detected by western blotting and SNA blotting. Actin was used as a loading control. The data are the mean ± SD of three independent assays (**p < 0.01, *p < 0.05). (B) The cells were pretreated with 1 μM BACE1 inh. for 24 h and then stimulated with TNF-α (50 ng/ml) for 24 h and analyzed by western blotting and SNA blotting. The data are the mean ± SD of three independent assays (**p < 0.01). ns, not significant. (C) The cells were pretreated with 1 μM BACE1 inh. for 24 h and then stimulated with TNF-α (50 ng/ml) for 24 h. The cells were incubated with FITC-labeled SNA and DAPI for 1 h. Proteins α-2, 6 sialylation levels were examined under confocal fluorescence microscopy (600 × magnification). (D) EA.hy926 cells were inoculated with a BACE1-overexpressing lentivirus and selected with puromycin for 4 weeks. BACE1, ST6Gal-I and total protein α-2, 6 sialylation levels were then detected by western blotting and SNA blotting. EA.hy926/Mock and EA.hy926 cells transduced with control lentivirus. EA.hy926/BACE1 and EA.hy926 cells transduced with BACE1-overexpressed lentivirus. (E) Total protein α-2, 6 sialylation levels in cells overexpressing BACE1 were examined by SNA staining. Because they were transduced with lentiviruses carrying an EGFP marker gene, the EA.hy926 cells were sequentially incubated with biotinylated SNA and Avidin-RBITC, which specifically binds biotinylated SNA. RBITC fluorescence signals were detected under confocal fluorescence microscopy. (F) Sialylated VE-cadherin levels were decreased after BACE1 overexpression. Cell lysates were immunoprecipitated with anti-VE-cadherin antibody and analyzed by SNA blotting. The data are the mean ± SD of three independent assays (**p < 0.01).