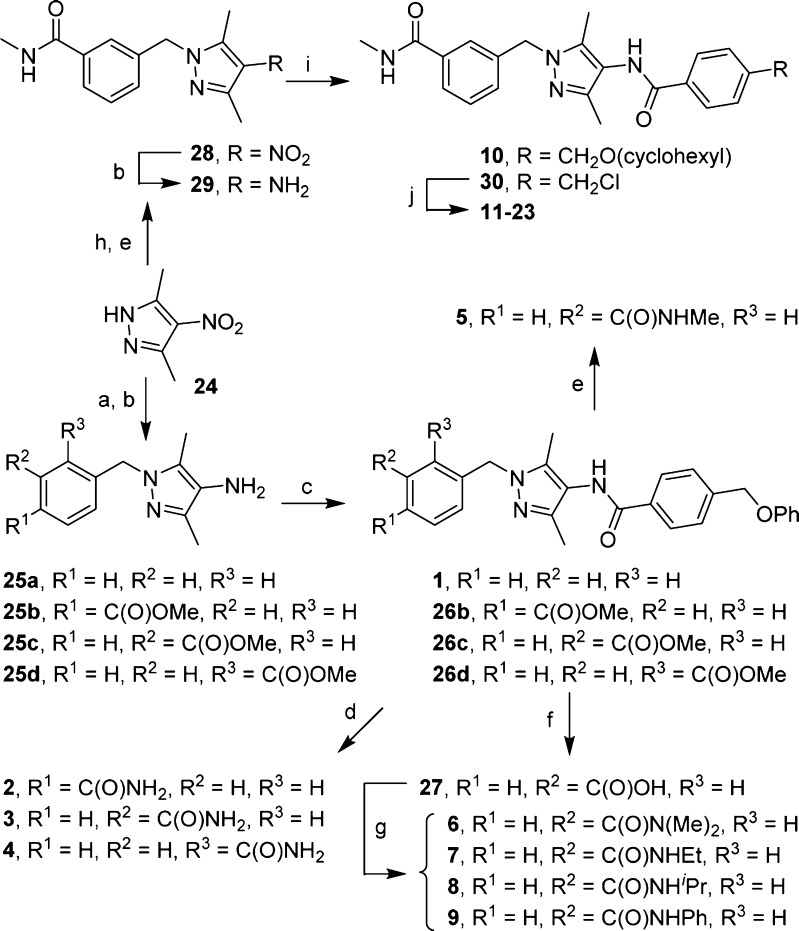
Scheme 1.
Reagents and conditions: (a) ArCH2Br, Cs2CO3, DMF; (b) NaBH4, NiCl2·6H2O, MeOH; (c) 4-(phenoxymethyl)benzoic acid, EDC, DMF/CH2Cl2; (d) 7 N methanolic NH3, 70 °C; (e) MeNH2, EtOH, 70 °C; (f) NaOH, MeOH, H2O; (g) amine, HBTU, Et3N, DMF/CH2Cl2; (h) methyl 3-(bromomethyl)benzoate, Cs2CO3, DMF; (i) for 10, 4-((cyclohexyloxy)methyl)benzoic acid, HBTU, Et3N; for 30, 4-(chloromethyl)benzoyl chloride, Et3N, CH2Cl2; (j) ArOH, Cs2CO3, DMF, 50 °C.