Abstract
With only two new classes of antibiotics developed in the last 40 years, novel antibiotics are desperately needed to combat the growing problem of multidrug-resistant and extensively drug resistant bacteria, particularly Gram-negative bacteria. Described in this letter is the synthesis and antibiotic activity of 1,2,4-triazolidine-3-thiones as narrow spectrum antibiotics. Optimization of the 1,2,4-triazolidine-3-thione scaffold identified a small molecule with potent antibiotic activity against multiple strains of multidrug-resistant and extensively drug-resistant Acinetobacter baumannii. This small molecule also shows single dose, in vivo activity in a Galleria mellonella infection model with A. baumannii and represents a promising start in the development of a class of drugs that can target this bacterial pathogen.
Keywords: antibiotic resistance, antibiotics, Acinetobacter baumannii
Antibiotic resistance has become one of the forefront issues in global health because of the emergence of multidrug-resistant (MDR) bacteria. According to the Centers for Disease Control and Prevention (CDC), an estimated two million people each year acquire MDR bacterial infections, of which 23,000 are fatal.1 Even more alarming is the dearth of antibiotic options emerging to treat these infections. Only two antibiotics belonging to novel structural classes have been brought into the clinic in the past 40 years, daptomycin and linezolid.2 Of great concern is that both of these antibiotics are only effective against Gram-positive bacteria, which leaves treatment options for MDR Gram-negative bacteria as an urgent unmet medical need. Prominent Gram-negative pathogens include Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species, which along with the Gram-positive species Enterococcus faecium and Staphylococcus aureus make up the bacterial species that are often referred to as “ESKAPE” pathogens.3 Currently, the most effective option to treat MDR Gram-negative bacteria remains the polymyxin colistin, which has significant side effects including nephrotoxicity.4 These side effects, coupled with colistin’s effectiveness, have made it a last resort antibiotic against MDR Gram-negative bacteria. Worryingly, colistin-resistant strains of bacteria are being isolated with greater frequency, and recently, a colistin-resistance gene was found on a plasmid that could easily move between bacterial species.5 Therefore, the threat of superbugs, bacteria becoming extensively drug resistant (XDR) and pandrug-resistant (PR), is a real possibility. As a result, new antibiotics with activity against Gram-negative pathogens are sorely needed.
The development of narrow spectrum antibiotics has some advantages over the traditional approach of developing broad-spectrum antibiotics, namely, the possibility of slower resistance generation and a reduction in detrimental effects on the gut microbiome including a decreased risk of antibiotic-associated colitis.6 As more rapid and sensitive diagnostic tests are developed, the use of narrow-spectrum antibiotics is becoming more feasible, and the EMA and the FDA have recently included antibacterial compounds active against a narrow spectrum of MDR pathogens in a list of treatments that qualify for a shorter route to registration.7 Of the Gram-negative ESKAPE pathogens, A. baumannii has recently come under the microscope because of its high mortality rates in the ICU (>50%), prevalence in wound infections in U.S. servicemen that have been injured in the conflicts in Iraq and Afghanistan,8 as well as its ability to survive in hospital environments and the isolation of PR strains.9
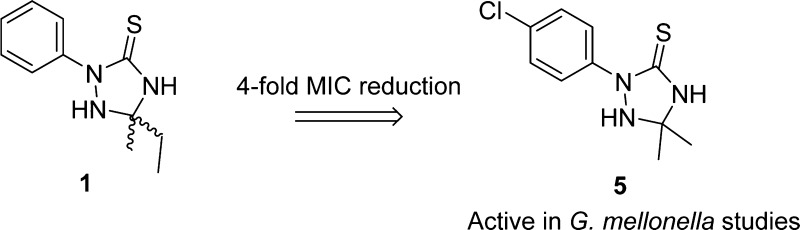
In 2009 compound 1 (Scheme 1) was reported to have antifungal activity against Candida albicans.10 Recently, genomic sequencing has revealed that A. baumannii harbors genes that share homology to a long chain fatty acid elongation pathway in fungi that was the supposed target of compound 1. Testing of 1,2,4-triazolidine-3-thiones, for antibiotic activity against A. baumannii identified compound 1 as a promising antibacterial lead.11 Using this compound as a structural template, we have synthesized analogues of the 1,2,4-triazolidine-3-thione core structure with variations introduced at the N-1, N-2, and C-5 positions as well as alkylations of the sulfur atom at C-3 in an attempt to identify new, more biologically active compounds. Herein, we report the synthesis of analogues of 1,2,4-triazolidine-3-thiones as well as their antibiotic activity against MDR-A. baumannii. Moreover, we report a compound that has minimum inhibitory concentrations (MICs) of up to 4-fold lower against multiple strains of MDR-A. baumannii compared to compound 1 and, unlike parent compound 1, shows activity in a Galleria mellonella model of infection.
Scheme 1. Synthesis of 1,2,4-Triazolidine-3-thiones12,13.
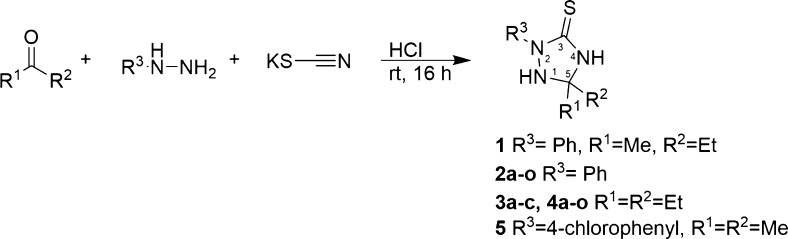
The 1,2,4-triazolidine-3-thione scaffold is readily accessible through a three-component reaction between ketones or aldehydes, hydrazines, and potassium thiocyanate in hydrochloric acid for 16 h in the dark (Scheme 1). The resulting triazolidines typically precipitate during the reaction, allowing for simple filtration followed by recrystallization from methanol to deliver purified material.12,13 Moreover, this synthetic procedure easily allows for modifications to be made at the N-2 and C-5 positions on the ring, thus allowing rapid assessment of the structure–activity relationship of 1,2,4-triazolidine-3-thiones.
Initially, we evaluated the effect that substitution at carbon 5 had upon activity by varying the identity of the aliphatic substituents. A series of 14 compounds that were mono- or di-substituted with alkyl or aryl groups were synthesized and screened for antibiotic activity against AB5075 under standard CLSI broth microdilution conditions14 (Table 1). A. baumannii strain AB5075 is an XDR primary clinical isolate and was chosen to evaluate the 1,2,4-triazolidine-3-thiones because of its virulence and XDR properties.15
Table 1. MIC Values for C-5 Modifications against AB5075, Where R3 = Phenyl.
| compd | R1 | R2 | MICa |
|---|---|---|---|
| 1 | methyl | ethyl | 8 |
| 2a | methyl | methyl | 8 |
| 2b | methyl | isopropyl | 8 |
| 2c | methyl | phenyl | >128 |
| 2d | methyl | H | >128 |
| 2e | ethyl | ethyl | 8 |
| 2f | ethyl | isopropyl | 32 |
| 2g | ethyl | H | >128 |
| 2h | n-propyl | methyl | 16 |
| 2i | n-propyl | ethyl | 32 |
| 2j | n-propyl | n-propyl | 32 |
| 2k | n-propyl | H | 64 |
| 2l | phenyl | H | 64 |
| 2m | cyclopentyl | 8 | |
| 2n | cyclohexyl | 8 |
All concentrations are in μg/mL.
Compound 1 exhibited an MIC of 8 μg/mL against AB5075, and both the dimethyl (compound 2a) and diethyl (compound 2e) derivatives exhibited identical activity to the parent compound. All other modifications at the C-5 position yielded either no change, or an increase in MIC compared to the parent compound (Table 1).
As we did not observe an increase in antibiotic activity through modification of the 5-position we next shifted our focus to the substitution at the N-2 position of the ring. The diethyl substituted derivatives were chosen instead of the parent methyl ethyl substituted derivatives to avoid racemic mixtures. Benzyl, cyclohexyl, and 2-pyridinyl substitutions of the N-2 position completely abolished biological activity (supporting information), which led to the conclusion that the phenyl ring at the N-2 position was required for activity (compound 3, Scheme 1).
Given the significant impact that changing the 2-phenyl substituent to a 2-benzyl had upon activity, we then investigated whether substitution of the phenyl ring itself had any effect on the MIC (Table 3). The 4-chloro derivative 4a exhibited increased activity compared to the parent compound, with an MIC of 2 μg/mL. Encouraged by this result, additional analogues were synthesized with various halogen substitutions in different positions on the phenyl ring. Unfortunately, only a decrease in activity was observed compared to the 4-chloro compound. Other substitutions on the phenyl ring were also made, but only halogen substitutions were tolerated (Table 2).
Table 3. MIC Results for Compounds 1, 4a, and 5 against Multiple MDR Strains of A. baumanniia.
| compd | AB-4878 | AB-3785 | AB-3806 | AB-4957 | BAA-1605 | AB-3638 |
|---|---|---|---|---|---|---|
| 1 | 8 | 4 | 4 | 8 | 8 | 4 |
| 4a | 4 | 4 | 4 | 4 | 4 | 4 |
| 5 | 2 | 1 | 1 | 2 | 2 | 1 |
The resistance profiles of these strains have been previously published.19 All concentrations are in μg/mL.
Table 2. MIC Values for Various Phenyl Ring Substitutions against AB5075, Where R1 = R2 = Ethyl.
| compd | R3 | MICa |
|---|---|---|
| 4a | (4-chlorophenyl) | 2 |
| 4b | (4-bromophenyl) | 4 |
| 4c | (4-iodophenyl) | 8 |
| 4d | (4-fluorophenyl) | 4 |
| 4e | (2-fluorophenyl) | 32 |
| 4f | (3-fluorophenyl) | 8 |
| 4g | (2-chlorophenyl) | 128 |
| 4h | (3-chlorophenyl) | 8 |
| 4i | (3-chloro-4-fluoro) | 32 |
| 4j | (3,5-difluorophenyl) | >128 |
| 4k | (3,4-dichlorophenyl) | >128 |
| 4l | (4-isopropylphenyl) | >128 |
| 4m | (4-nitrophenyl) | >128 |
| 4n | (4-cyanophenyl) | >128 |
| 4o | (4-trifluoromethylphenyl) | >128 |
All concentrations are in μg/mL.
With a more active derivative 4a identified, we next tested the lead compound and parent compound against multiple strains of MDR-A. baumannii (representative examples shown in Table 3). The dimethyl analogue of compound 4a, compound 5(16−18) (Scheme 1), was also prepared to investigate whether the lipophilicity of the compounds could be tuned without affecting the activity of the compounds (note: our spectral data for compound 5 did not match previously reported data; however, the crystal structure of our synthetic sample was determined to unambiguously establish identity). Compound 4a has a predicted log D value of 3.85, while compound 5 has a predicted log D value of 2.58 at pH 7.
All three compounds exhibited activity against multiple strains of A. baumannii, while compound 5 was more active against several strains of A. baumannii than either compound 4a or the parent compound 1 (Table 3). Additional MIC results against more strains of A. baumannii can be found in the Supporting Information.
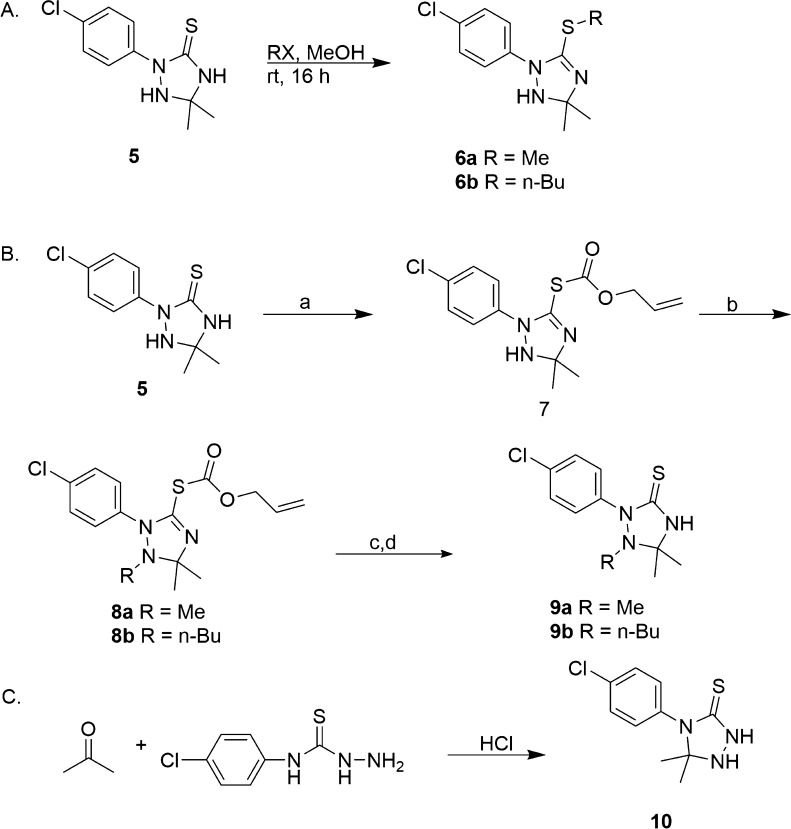
After the identification of compound 5 as the most active analogue against several A. baumannii strains, we next opted to investigate further structure–activity relationships of compound 5, beginning with the effect of alkylating the sulfur atom at the C-3 position. Briefly, compound 5 was reacted with the corresponding alkyl halide in methanol at room temperature overnight to yield the thioethers 6a–b (Scheme 2). The methyl and benzyl analogues were prepared, and the observed MIC for both compounds was greater than 128 μg/mL, showing the necessity of the unsubstituted sulfur atom for biological activity. To further probe the SAR of the 1,2,4-triazolidine-3-thione core, effect of alkylation of the N-1 position of compound 5 was also investigated (Scheme 2).
Scheme 2. Synthetic Routes To Allow for the Alkylation of the Thiol Moiety (A), N-1 Position (B), and Move of 4-Chloro Substituent (C) of Compound 5.
Reagents and conditions: (a) Alloc-Cl, DMAP, Et3N, THF rt, 16 h; (b) RX, NaH, DMF 0° C to rt, 4 h; (c) NaBH4, Pd(PPh3)4, EtOH 0° C to rt, 1 h; (d) 12 N HCl (pH 2.5-3), rt, 4 h.
In order to alkylate the N-1 position, the thiol was protected with an alloc protecting group. The N-1 position was then alkylated using sodium hydride and the corresponding alkyl halide. The alloc group was removed using sodium borohydride and tetrakis(triphenylphosphine)palladium(0) in ethanol at 0 °C, and the solution was then acidified with 12 N HCl at room temperature.20 The MICs of compounds 9a–b were all greater than128 μg/mL, demonstrating the need for the free N–H at the N-1 position on the 1,2,4-triazolidine-3-thione ring for biological activity.
The final structural modification that was investigated was to move the 4-chlorophenyl substituent from the N-2 position to the N-4 position of compound 5 (Scheme 2C). The reverse 1,2,4-triazolidine-3-thione scaffold is accessed through the reaction of N-(4-chlorophenyl) hydrazinecarbothioamide with acetone in hydrochloric acid for 16 h in the dark (Scheme 2C). As with compounds 1–4o, the resulting triazolidine precipitated during the reaction, allowing for simple filtration followed by recrystallization from methanol to deliver purified material.12,13 The observed MIC of compound 10 was greater than 128 μg/mL, which is a greater than 64-fold increase in MIC compared to compound 5, indicating that the original substitution pattern of the 1,2,4-triazolidine-3-thiones is the more active motif.
We next investigated whether compounds 5 was acting in a bactericidal or bacteriostatic manner by constructing a time kill curve. An antibiotic is defined as bactericidal when it kills ≥99.9% of bacteria at a concentration no greater than four times the MIC.21 As seen in the time-kill curve below (Figure 1), compound 5 is bactericidal, effecting a greater than six log reduction in colony forming units per milliliter (CFU/mL) at double the MIC and greater.
Figure 1.
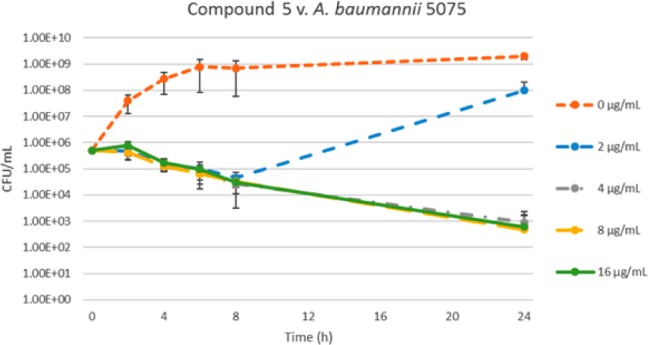
Time-kill curve for compound 5 against AB5075.
In C. albicans and A. baumannii, compound 1 distorts the ratio of unsaturated to saturated fatty acids.10,11 It was also established that fatty acid supplementation of media abrogated the bactericidal effects of compound 1 against A. baumannii.11 In order to test whether compound 5 exhibited similar behavior, an MIC assay under fatty acid supplementation was performed. As with compound 1, when the media was supplemented with 0.02% linoleic acid, the activity of compound 5 against AB5075 was completely abolished with an observed MIC of >128 μg/mL.
In order to probe the antibiotic spectrum of compound 5, MICs against Escherichia coli, P. aeruginosa, K. pneumoniae, and methicillin resistant S. aureus (MRSA) were recorded. An MIC of ≥128 μg/mL was observed for all bacterial species other than A. baumannii, establishing that as with the original lead 1, compound 5 is a narrow spectrum antibiotic. The synergistic potential of compound 5 with other antibiotics was also investigated utilizing checkerboard assays.22 Compound 5 did not show synergy with Meropenem or colistin against AB5075, or with colistin against the colistin-resistant AB3941.
Given the potential hydrolytic instability of 5, we performed a time-dependent stability study. Compound 5 was dosed in cation adjusted Mueller–Hinton Broth (CAMHB) and incubated at 37 °C. The mixture was then analyzed by taking liquid chromatography. We determined that compound 5 has a half-life of about 2.5 h in these conditions and is almost fully degraded after 16 h (Supporting Information). After observing the decomposition of the compound, compound 5 was dosed in CAMHB at 4 μg/mL and incubated for 1, 2, 4, and 8 h. The activity of each of the incubated solutions was then tested against A. baumannii 5075, and compound 5 was found to have no activity at its MIC after incubation in MHB for any of the time points. This indicates that compound 5, and not any degradation products, is most likely the active chemical species.
The toxicity of compound 5 to eukaryotic cell membranes was also investigated by measuring its hemolytic activity against mechanically defibrinated sheep blood as previously reported.23 Compound 5 did not lyse the red blood cells at any concentration tested (up to 512 μg/mL, Supporting Information).
Finally, we compared the activity of compound 1 and 5 in a G. mellonella infection model with AB5075.15 Compound 1 was inactive against AB5075 in this model (data not shown, Supporting Information). However, compound 5 exhibited modest in vivo activity, with 50 mg/kg of compound 5 saving 22% of worms after 6 days, and 100 mg/kg saving 32% of worms after 6 days. In comparison, 100% of untreated, infected worms were dead after 6 days (Figure 2). The comparison is more stark at 3 days, where almost 90% of the worms are dead in the untreated group, but in contrast, 42.5% and 45% survival are seen with the 50 mg/kg and 100 mg/kg treatments, respectively (Figure 2).
Figure 2.
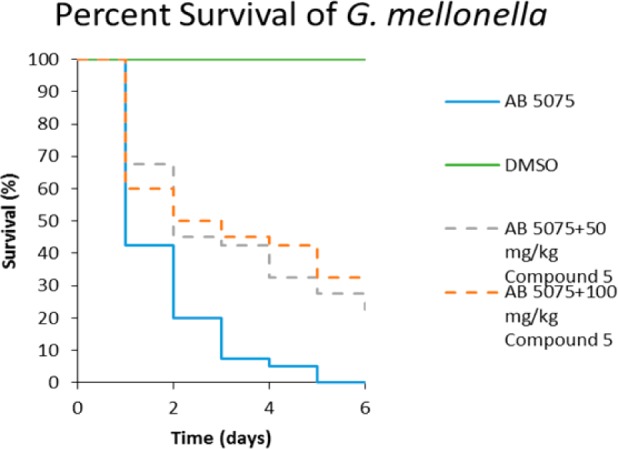
Percent survival of G. mellonella after AB5075 infection and compound 5 treatment.
In conclusion, following the identification of the 1,2,4-triazolidine-3-thiones as possessing antibiotic activity against A. baumannii we synthesized a diverse array of analogues in attempts to identify a compound with increased activity. After making modifications at the C-5 and N-2 positions on the 1,2,4-triazolidine-3-thione core structure to no effect, substitutions were made to the phenyl ring at position 2 to yield compound 4a, which displayed a 4-fold improvement in MIC against A. baumannii compared to compound 1. Compound 5 was also synthesized and tested against multiple strains of A. baumannii along with compound 1 and compound 4a in order to ascertain whether the lipophilicity could be tuned without affecting the MIC. Compound 5 displayed an up to 4-fold improvement against multiple strains of A. baumannii compared with compound 1, as well as returning a lower MIC than compound 4a in most cases. Compound 5 was found to be bactericidal, and an in vivo study showed positive survival results when compound 5 was used against AB5075 in a G. mellonella model of infection. While these results were modest, it is important to note that just a single dose was provided, and most antibiotics are dosed multiple times a day. Furthermore, AB5075 is a more virulent strain than most other A. baumannii isolates,14 which provides a higher bar when evaluating activity. Future studies are now underway to develop structural modifications and conduct biological evaluations against A. baumannii using compound 5 as our current lead in an attempt to further augment in vitro activity and improve in vivo results.
Acknowledgments
Dr. Zurawski would like to thank the Multidrug-resistant Organism Repository and Surveillance Network (MRSN) for providing the isolates used in this study.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.6b00296.
Compound characterization for all novel compounds, 1H and 13C NMR spectra, MIC and full in vivo testing data (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors from N.C. State would like to thank the university for support. The authors from Wound Infection Department at WRAIR would like to acknowledge the constant support of the Military Infectious Diseases Research Program.
The material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the authors, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense.
The authors declare no competing financial interest.
Supplementary Material
References
- CDC. Antibiotic resistance threats in the United States, 2013.
- Clatworthy A. E.; Pierson E.; Hung D. T. Targeting virulence: a new paradigm for antimicrobial therapy. Nat. Chem. Biol. 2007, 3 (9), 541–8. 10.1038/nchembio.2007.24. [DOI] [PubMed] [Google Scholar]
- Boucher H. W.; Talbot G. H.; Bradley J. S.; Edwards J. E.; Gilbert D.; Rice L. B.; Scheld M.; Spellberg B.; Bartlett J. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48 (1), 1–12. 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- Falagas M. E.; Kasiakou S. K. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect. Dis. 2005, 40 (9), 1333–41. 10.1086/429323. [DOI] [PubMed] [Google Scholar]
- Liu Y. Y.; Wang Y.; Walsh T. R.; Yi L. X.; Zhang R.; Spencer J.; Doi Y.; Tian G.; Dong B.; Huang X.; Yu L. F.; Gu D.; Ren H.; Chen X.; Lv L.; He D.; Zhou H.; Liang Z.; Liu J. H.; Shen J. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16 (2), 161–8. 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- Maxson T.; Mitchell D. A. Targeted Treatment for Bacterial Infections: Prospects for Pathogen-Specific Antibiotics Coupled with Rapid Diagnostics. Tetrahedron 2016, 72 (25), 3609–3624. 10.1016/j.tet.2015.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bax R.; Green S. Antibiotics: the changing regulatory and pharmaceutical industry paradigm. J. Antimicrob. Chemother. 2015, 70 (5), 1281–1284. 10.1093/jac/dku572. [DOI] [PubMed] [Google Scholar]
- O’Shea M. K. Acinetobacter in modern warfare. Int. J. Antimicrob. Agents 2012, 39 (5), 363–75. 10.1016/j.ijantimicag.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Sunenshine R. H.; Wright M. O.; Maragakis L. L.; Harris A. D.; Song X.; Hebden J.; Cosgrove S. E.; Anderson A.; Carnell J.; Jernigan D. B.; Kleinbaum D. G.; Perl T. M.; Standiford H. C.; Srinivasan A. Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerging Infect. Dis. 2007, 13 (1), 97–103. 10.3201/eid1301.060716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D.; Sillaots S.; Davison J.; Hu W.; Jiang B.; Kauffman S.; Martel N.; Ocampo P.; Oh C.; Trosok S.; Veillette K.; Wang H.; Yang M.; Zhang L.; Becker J.; Martin C. E.; Roemer T. Chemical genetic profiling and characterization of small-molecule compounds that affect the biosynthesis of unsaturated fatty acids in Candida albicans. J. Biol. Chem. 2009, 284 (29), 19754–64. 10.1074/jbc.M109.019877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey B. W.; Thompson M. G.; Hittle L. E.; Jacobs A. C.; Asafo-Adjei E. A.; Huggins W. M.; Melander R. J..; Melander C.; Ernst R. K.; Zurawski D. V. 1,2,4 -Triazolidine-3-thiones have specific activity against Acinetobacter baumannii amongst common nosocomial pathogens. ACS Infect. Dis. 2016, 10.1021/acsinfecdis.6b00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai I. Reactions of Phenylhydrazinium Thiocyanate with Ketones and Aldehydes. Bull. Chem. Soc. Jpn. 1973, 46 (7), 2215–2218. 10.1246/bcsj.46.2215. [DOI] [Google Scholar]
- Lagoja I. M.; Pannecouque C.; Musumeci L.; Froeyen M.; Van Aerschot A.; Balzarini J.; Herdewijn P.; De Clercq E. 1,2,4-Triazole Derivatives Inhibiting the Human Immunodeficiency Virus Type 1 (HIV-1) in vitro. Helv. Chim. Acta 2002, 85, 1883–92. . [DOI] [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement M100-S22, 2012.
- Jacobs A. C.; Thompson M. G.; Black C. C.; Kessler J. L.; Clark L. P.; McQueary C. N.; Gancz H. Y.; Corey B. W.; Moon J. K.; Si Y.; Owen M. T.; Hallock J. D.; Kwak Y. I.; Summers A.; Li C. Z.; Rasko D. A.; Penwell W. F.; Honnold C. L.; Wise M. C.; Waterman P. E.; Lesho E. P.; Stewart R. L.; Actis L. A.; Palys T. J.; Craft D. W.; Zurawski D. V. AB5075, a Highly Virulent Isolate of Acinetobacter baumannii, as a Model Strain for the Evaluation of Pathogenesis and Antimicrobial Treatments. mBio 2014, 5 (3), e01076–14. 10.1128/mBio.01076-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Toro V.; Gozzo F.; Lorusso S.; Garavaglia C.. Fungicidal preparations containing derivatives of 1,2,4-triazolidion-3-ones or -thiones. 1979.
- Schantl J.; Hebeisen P. 2-Aryl-5,5-dimethyl-1,2,4-triazolidin-3-one derivatives. Sci. Pharm. 1983, 51, 379–90. [Google Scholar]
- Schantl J. G.; Hebeisen P.; Minach L. α-Arylazoalkyl isocyanates and isothiocyanates by potassium permanganate oxidation of 2,5,5-trisubstituted 1,2,4-triazolidin-3-ones and 1,2,4-triazolidin-3-thiones. Synthesis 1984, 4, 315–317. 10.1055/s-1984-30823. [DOI] [Google Scholar]
- Taitt C. R.; Leski T. A.; Stockelman M. G.; Craft D. W.; Zurawski D. V.; Kirkup B. C.; Vora G. J. Antimicrobial resistance determinants in Acinetobacter baumannii isolates taken from military treatment facilities. Antimicrob. Agents Chemother. 2014, 58, 767–781. 10.1128/AAC.01897-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvetovich R. J.; Kelly D. H.; DiMichele L. M.; Shuman R. F.; Grabowski E. J. J. Syntheses of 4″-epi-Amino-4″-deoxyavermectinB1. J. Org. Chem. 1994, 59, 7704–7708. 10.1021/jo00104a028. [DOI] [Google Scholar]
- French G. L. Bactericidal agents in the treatment of MRSA infections--the potential role of daptomycin. J. Antimicrob. Chemother. 2006, 58 (6), 1107–17. 10.1093/jac/dkl393. [DOI] [PubMed] [Google Scholar]
- Orhan G.; Bayram A.; Zer Y.; Balci I. Synergy tests by E test and checkerboard methods of antimicrobial combinations against Brucella melitensis. J. Clin. Microbiol. 2005, 43 (1), 140–3. 10.1128/JCM.43.1.140-143.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington R. J.; Bunders C. A.; Reed C. S.; Melander C. Small molecule suppression of carbapenem resistance in NDM-1 producing Klebsiella pneumoniae. ACS Med. Chem. Lett. 2012, 3 (5), 357–361. 10.1021/ml200290p. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.