Abstract
This study aimed to evaluate the clinical variations in patients with Parkinson’s disease (PD) with (PDRBD) or without REM sleep behaviour disorder (RBD) (Non-RBD), and PDRBD patients were classified into Confirmed-RBD (definite diagnosis with polysomnography, PSG) and Probable-RBD (without PSG re-confirmation). The clinical difference between the groups of patients was measured as an odds ratio (OR) or standardized mean difference (SMD, Cohen d). A total of 31 articles with data from 5,785 participants were obtained for our analysis. Overall, the occurrence of Confirmed-RBD was more frequent in male patients (OR = 1.25; p = 0.038), elderly patients (SMD = 0.25; p = 0.000), and patients with longer disease duration (SMD = 0.30; p = 0.000), increased Hoehn-Yahr scale (SMD = 0.30; p = 0.000), and higher UPDRS-III score (SMD = 0.38; p = 0.002). On the other hand, the frequency of Probable-RBD was increased with disease duration (SMD = 0.29; p = 0.000), Hoehn-Yahr scale (SMD = 0.30; p = 0.000), and UPDRS-III score (SMD = 0.26; p = 0.001). Our study indicate that PDRBD patients may have different clinical features compared to patients with Non-RBD.
REM sleep behaviour disorder (RBD), a parasomnia that is characterized by a loss of normal skeletal muscle atonia during REM sleep and elaborate motor activity associated with oneiric content, is one of the most common non-motor symptoms of Parkinson’s disease (PD)1,2. Epidemiological investigation demonstrated that RBD, which has a prevalence of <1% in the general population3,4, is a common disorder that occurs in 15–75% of patients with PD, especially males over 50 years of age5,6,7,8. RBD frequently leads to serious injuries to PD patients, e.g., falling out of bed, and patients can harm their sleeping partner while dreaming1,9.
RBD is considered a heralding disorder or early marker of α-synucleinopathies, including dementia with Lewy bodies (DLB), multiple system atrophy (MSA), and Parkinson’s disease (PD)10,11,12. A longitudinal cohort study revealed that RBD appears several years or even decades before the onset of the motor symptoms of PD13. Furthermore, 38% of patients developed a parkinsonian syndrome approximately 3.7 years after an initial diagnosis of idiopathic RBD10.
Current research suggests that neuronal degeneration of brainstem nuclei, including the pontine tegmental area and medulla, is the pathophysiological cornerstone of RBD14. However, there are still a remarkable number of PD patients who do not display RBD symptoms, even with long disease duration. Thus, there are likely additional factors involved in the occurrence of PD-related RBD (PDRBD) other than neuronal degeneration.
Numerous studies are available on the prevalence, clinical characteristics and disease staging of RBD. However, systemic meta-analyses in large samples for comparing the clinical variations between patients with PDRBD and those with PD alone (Non-RBD) are scarce. Therefore, the primary aims of our study were to compare the clinical characteristics of PDRBD and Non-RBD. This study was conducted with data originating from multiple studies with an overall moderate quality.
Methods
Data sources and search strategy
The study protocol was approved by the Clinical Ethics Committee of The First Affiliated Hospital of Anhui Medical University. In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement15, we undertook a systematic search of PubMed and Embase databases for studies published between January 2000 and August 2016 with a language limitation of English only. The search terms “rapid eye movement”, “rem”, “rem sleep behavior disorder”, or “rapid eye movement sleep behavior disorder” were combined using the Boolean logical operator AND with studies identified by the terms “parkinson”, “parkinson’s”, “parkinson disease”, “parkinson’s disease”, “parkinsonian”, “parkinsonian disease” or “parkinsonian disorders”. The references in the primary selected articles, relevant reviews and meta-analyses were also examined to identify additional relevant studies.
Data extraction and quality assessment
Prospective or retrospective case-control studies that compared PD patients with and without RBD were eligible for inclusion in our meta-analysis. The papers were initially screened on the basis of their titles and/or abstracts by two reviewers (Ruo-lin Zhu and Cheng-juan Xie). For inclusion in the meta-analysis, a study had to meet the following criteria: (1) the original data were published; (2) the diagnosis of PD was confirmed with the U.K. Parkinson’s Disease Society Brain Bank criteria or other criteria; (3) the PD incidence data were classified into groups of PDRBD and Non-RBD. Notably, in this meta-analysis, a definition of Confirmed-RBD was made when RBD patients met the criteria described in the International Classification of Sleep Disorders (ICSD), in which polysomnography (PSG) is mandatory; otherwise, the Probable-RBD was defined in RBD patients who were diagnosed based on interview or questionnaires. (4) the study described the number of participants and PD cases, ratio or number of male/female subjects, age, onset age of PD, disease duration, Hoehn-Yahr staging scale for disease activity, and the Unified Parkinson’s Disease Rating Scale part III (UPDRS-III) score for motor symptom severity. Studies with inadequate data on the diagnosis of PD and/or RBD and those focused on pathogenic mechanisms were excluded. Articles that included fewer than 20 patients and studies based on animal research were also excluded.
Bias risk and applicability were critically evaluated based on a quantitative 5-point Jadad scale16. The Jadad scale focuses on 4 critical items, including randomisation (maximum of 2 points), blinding (maximum of 2 points) and an account of all patients (maximum of 1 point). The Jadad scores were determined by two reviewers (Ruo-lin Zhu and Cheng-juan Xie). Scoring disagreements were determined by an arbitrator (Kai Wang).
Statistical analysis
The meta-analysis of dichotomous or continuous variables was conducted using the Mantel-Haenszel method with a fixed- or random-effects model (based on the heterogeneity analysis). Data were combined and expressed as odds ratios (ORs) or standardized mean differences (SMDs) for dichotomous and continuous variables, respectively, with 95% confidence intervals (95% CI).
Forest plots were presented with a measure of inconsistency across the trials (the Cochrane Q statistic was calculated from Chi2 and the I2 statistic) and a test for overall effects (Z). The statistical heterogeneity between studies was assessed with the Cochran’s Q-test and I2-values. If the Cochran’s Q test had a p value < 0.05, an I2 value over 75% was defined as high inconsistency, an I2 above 50% was defined as moderate heterogeneity, and an I2 below 25% was defined as low levels of inconsistency amongst the included studies17,18,19.
Funnel plots were produced to detect potential publication bias. All statistical tests and the construction of forest plots were performed using Stata/SE version 13.1 (StataCorp, College Station, TX, USA). The significance level was set at p < 0.05.
Results
Study identification and methodological quality
The initial search strategy yielded 3,341 potentially relevant studies from the PubMed (n = 1,117) and Embase (n = 2,224) databases. After 1,159 duplicates were removed, the titles and/or abstracts of 2,182 citations were reviewed, and 839 studies were excluded. The remaining 1,343 full-text articles were retrieved for further evaluation. Another 1,187 studies were excluded because they were reviews (n = 655), case reports (n = 166), animal studies (n = 306) or editorials (n = 50). Ultimately, 31 articles1,2,7,8,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46 were included in our analysis (Fig. 1). The diagnosis of Confirmed-RBD and Probable-RBD were defined in 19 and 12 studies respectively (Table 1). No disagreements on study selection occurred between the reviewers.
Figure 1. Study flow chart.
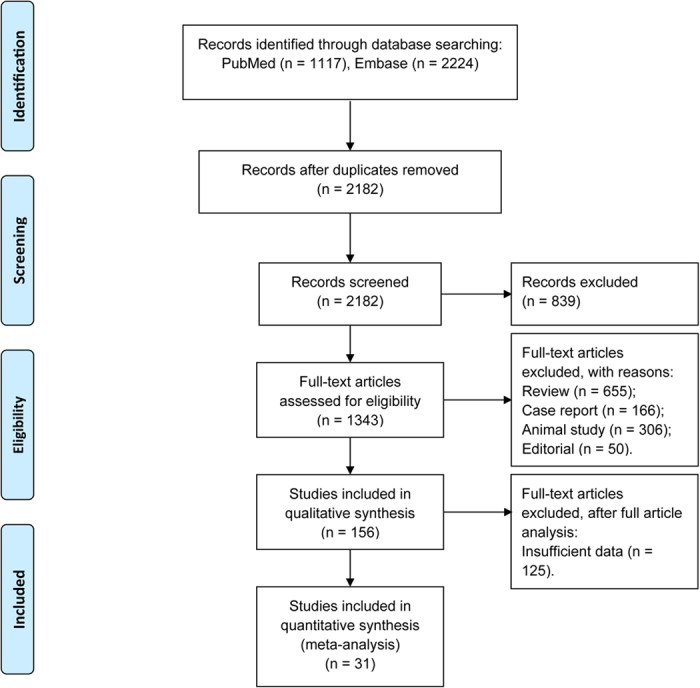
Table 1. Demographic and clinical characteristics of patients with Parkinson’s disease in the included studies.
| Year | Author | Source | Diagnostic criteria for PD; Disease Characteristics | Diagnosis tool for RBD | Participants (n) | Age (mean ± SD, year) | Gender (M/F) | Jadad score |
|---|---|---|---|---|---|---|---|---|
| Confirmed RBD | ||||||||
| 2015 | Arnulf et al. | France | PDSBB | ICSD | 114 | RBD: 62.8 ± 6.8 | 80/34 | 4 |
| NRBD: 56.8 ± 10.5 | ||||||||
| 2013 | Fabio et al. | Switzerland | NR | PSG + RBDSQ (≥6) | 20 | RBD: 65.5 ± 7.3 | 13/7 | 4 |
| NRBD: 65.8 ± 9.7 | ||||||||
| 2008 | Gjerstad et al. | Norway | Clinical criteria (Larsen 1994); probable RBD | ICSD | 245 | RBD: 71.6 ± 7.9 | 114/117 | 3 |
| NRBD: 73.7 ± 8.5 | ||||||||
| 2014 | Gong et al. | China | PDSBB | ICSD-2 | 120 | 66.16 ± 9.33 (44–83) | 70/42 | 4 |
| 2015 | Jankovic et al. | Serbia | PDSBB | ICSD-2 + RBDQ-HK | 97 | 62.1 ± 8.8 | 56/41 | 2 |
| 2014 | Kim et al. | Korea | PDSBB | ICSD-R | 944 | 63.67 ± 9.33 | 448/496 | 2 |
| 2015 | Kim et al. | Korea | NR | ICSD-R | 124 | 62.98 ± 7.87 (34–78) | 40/50 | 2 |
| 2009 | Lee et al. | Korea | PDSBB | ICSD-R | 447 | 63.8 ± 9.2 | 207/240 | 2 |
| 2016 | Lim et al. | Korea | PDSBB | PSG + RBDSQ-K (>5) | 38 | RBD: 69.8 ± 6.4 | 20/18 | 2 |
| NRBD: 69.7 ± 7.2 | ||||||||
| 2014 | Neikrug et al. | USA | NR | ICSD-2 + RBDSQ (≥5) | 183 | 67.2 ± 9.4 (47–89) | 43/19 | 4 |
| 2013 | Nomura et al. | Japan | PDSBB; Clinical and subclinical RBD | ICSD-2 | 82 | 74.3 ± 7.2 | 28/31†; | 4 |
| 22/33# | ||||||||
| 2012 | Postuma et al. | Canada | PDSBB | ICSD-2 | 42 | RBD: 70.5 ± 7.4 | 34/8 | 4 |
| NRBD: 67.5 ± 10.6 | ||||||||
| 2006 | Sinforiani et al. | Italy | PDSBB; PD at least 5 years | ICSD | 110 | NRBD: 63.03 ± 8.17; | RBD-1: 42/24 | 3 |
| RBD-1§: 66.03 ± 9.19; | RBD-2: 42/33 | |||||||
| RBD-2‡: 69.58 ± 7.42 | ||||||||
| 2011 | Sixel-Doring et al. | Germany | PDSBB | ICSD-2 | 463 | RBD: 69 ± 8 | 288/169 | 4 |
| NRBD: 66 ± 11 | ||||||||
| 2013 | Sorensen et al. | Denmark | NR | ICSD | 23 | RBD: 62.5 ± 6.9 | 14/9 | 3 |
| NRBD: 60.8 ± 6.7 | ||||||||
| 2007 | Vendette et al. | Canada | PDSBB; H-Y stage I-III | ICSD-2 | 34 | RBD: 65.61 ± 7.73 | NR | 4 |
| NRBD: 65.13 ± 7.69 | ||||||||
| 2011 | Vibha et al. | India | PDSBB | ICSD-R | 134 | RBD: 61.27 ± 8.56 | 90/44 | 2 |
| NRBD: 57.57 ± 11.85 | ||||||||
| 2009 | Yoritaka et al. | Japan | Clinical criteria (Calne, 1992); H-Y stage I–IV | ICSD-R | 150 | 68.5 ± 9.8 | 70/80 | 3 |
| 2016 | Zhang et al. | China | PDSBB; MMSE >21 | ICSD-2 | 46 | RBD: 66.71 ± 7.21 | 36/10 | 4 |
| NRBD: 62.46 ± 4.8 | ||||||||
| Probable RBD | ||||||||
| 2016 | Arnaldi et al. | Italy | Clinical criteria (Gelb 1999); de novo, drug-naïve PD | MSQ | 38 | RBD: 72.8 ± 6.2 | 24/14 | 3 |
| NRBD: 70.6 ± 7.1 | ||||||||
| 2012 | Boot et al. | USA | Mayo Clinic Study of Aging; UPDRS ≥4 | MSQ | 2050 | RBD: 78 (75, 82) | 455/196 | 2 |
| NRBD: 77 (74, 82) | ||||||||
| 2015 | Fantini et al. | France | PDSBB | RBD1Q + RBDSQ (≥6) | 216 | RBD: 67.3 ± 9.9 | 130/86 | 3 |
| NRBD: 66.4 ± 11.6 | ||||||||
| 2013 | Ford et al. | Australia | PDSBB | MSQ | 124 | RBD: 66.4 ± 9.9 | 84/40 | 4 |
| NRBD: 65.8 ± 10.9 | ||||||||
| 2015 | Hu et al. | China | PDSBB; Probable RBD | RBDSQ (≥6) | 225 | RBD: 61.19 ± 11.76 | 117/108 | 3 |
| NRBD: 59.79 ± 10.37 | ||||||||
| 2016 | Kim et al. | Korea | NR | RBDSQ-K (≥5) | 42 | 62.1 ± 8.8 | 18/24 | 2 |
| 2012 | Kotagal et al. | USA | PDSBB | MSQ (not confirmed by PSG) | 80 | RBD: 63.4 ± 6.7 | 60/20 | 3 |
| NRBD: 65.3 ± 7.1 | ||||||||
| 2014 | Mahale et al. | India | PDSBB | RBDSQ | 156 | 55.4 ± 11.2 | 119/38 | 2 |
| 2012 | Nihei et al. | Japan | PDSBB | RBDSQ-J | 469 | 71.0 ± 8.3 (44.4–91.8) | 219/250 | 3 |
| 2005 | Ozekmekci et al. | Turkey | NR; Movement disorders and injurious behavior during sleep | Clinical diagnostic criteria | 70 | RBD: 67.2 ± 8.6 | 54/16 | 2 |
| NRBD: 67.6 ± 9.19 | ||||||||
| 2013 | Poryazova et al. | Switzerland | Questionnaire on sleep-wake disorders and PD characteristics | RBDSQ (≥6) | 417 | 69 ± 9 | 273/131 | 3 |
| 2013 | Suzuki et al. | Japan | PDSBB; Probable RBD | RBDSQ-J (≥5) | 77 | 69.7 ± 8.9 | 40/37 | 4 |
RBD: rapid eye movement sleep behavior disorder; NRBD: Non-RBD; Confirmed-RBD: patients diagnosed according to the ICSD-2 criteria, with PSG confirmation; Probable-RBD: diagnosis based on interview/questionnaires, w/o PSG reconfirmation; PDSBB: U.K. Parkinson’s Disease Society Brain Bank criteria; MSQ: Mayo Sleep Questionnaire; H-Y: Hoehn-Yahr staging scale; ICSD: the International Classification of Sleep Disorders criteria for RBD; MMSE: Mini Mental State Examination; RBDSQ: the RBD screening questionnaire; PSG: Polysomnography.
§RBD-1: PD-RBD w/o hallucinations.
‡RBD-2: PD-RBD with hallucinations; NR: Not reported.
†Clinical RBD.
#Subclinical RBD.
The studies used for in our meta-analysis consisted of 7,380 randomised subjects, which ultimately encompassed 5,815 participants (PDRBD, n = 2,319, mean group size = 74.8; and Non-RBD, n = 3,496, mean group size n = 112.8) (Table 1). Fourteen studies were performed in Asia, 11 in Europe, 5 in North America, and 1 in Australia. The mean age across all subjects ranged from 54.1 to 76.5 years, and approximately 57.6% of patients were male. The onset age of PD ranged from 51.0 to 65.5 years. The mean disease duration of all participants ranged from 4.3–15.3 years. The mean Hoehn-Yahr scale and UPDRS-III score ranged from 0.9 to 3.2 and 0.23 to 190.97, respectively.
PDRBD patients were further classified into subgroups for two studies: clinical or subclinical RBD in the study conducted by Nomura et al.36 and PD-RBD with and without hallucinations in the study conducted by Sinforiani et al.40. The subgroup data were included independently in the meta-analysis for the variables of sex, age, disease duration, Hoehn-Yahr scale, and UPDRS-III score. The methodological quality was appraised for all studies included in the meta-analysis. Overall, the included studies were of moderate methodological quality according to the Jadad scale (Table 1).
Summary of effects
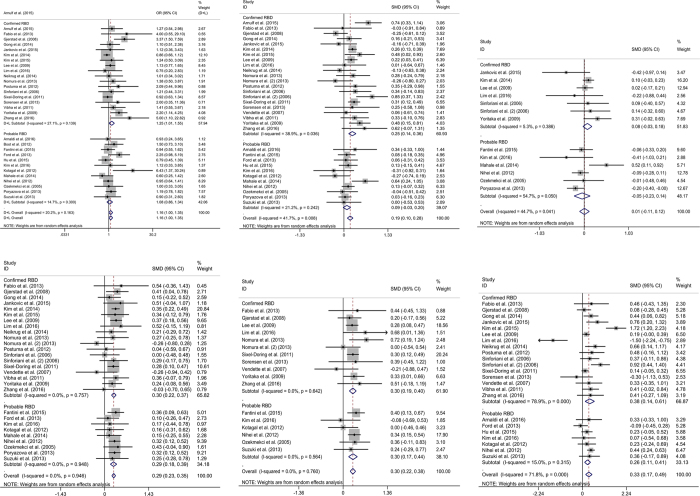
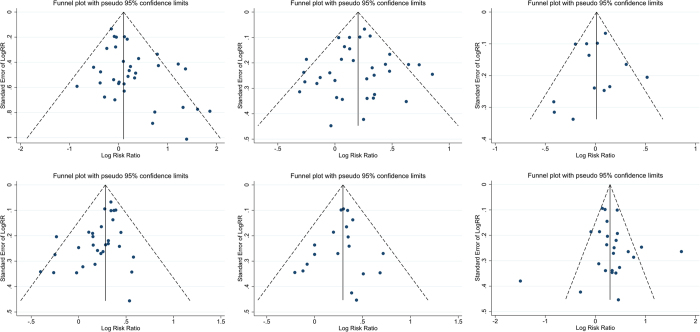
With regard to sex, a total of 5,685 PD patients, including 2,252 PDRBD patients and 3,433 Non-RBD patients, from 30 studies were analysed. The study by Vendette et al.43 was not included because the gender ratio or number was not reported. The proportion of male patients was 57.8% (males in PDRBD, n = 1,298 and males in Non-RBD, n = 1,988). High-quality evidence that showed male patients had an increased risk of RBD. Overall, male patients with PD were more likely to develop Confirmed-RBD than female patients (OR = 1.25; 95% CI 1.01–1.55; p = 0.038); on the other hand, our meta-analysis showed no significant impact of gender on the occurrence of Probable-RBD in PD patients (OR = 1.08; 95% CI 0.86–1.34; p = 0.516). There was moderate heterogeneity across studies for analysis of Confirmed-RBD (I2 = 27.1%; p = 0.139) and no heterogeneity for Probable-RBD (I2 = 14.7%; p = 0.300) (Fig. 2A). The funnel plot did not suggest substantial asymmetry (Fig. 3A).
Figure 2. Forrest plot of the odds ratios (ORs) and standardised mean differences (SMD, Cohen d) for the clinical variations between PD patients with and without RBD (PDRBD and Non-RBD, respectively).
If the Cochran’s Q test had a p value < 0.05, the I2 over 75%, above 50% and below 25% were defined as high, moderate and low inconsistency, respectively. (A) Gender, (B) patient age, (C) onset age of Parkinson’s disease, (D) disease duration, (E) Hoehn-Yahr staging scale, and (F) Unified Parkinson’s Disease Rating Scale part III (UPDRS-III) score.
Figure 3. Funnel plot with pseudo 95% confidence limits for assessment of publication bias.
(A) Gender, (B) patient age, (C) onset age of Parkinson’s disease, (D) disease duration, (E) Hoehn-Yahr staging scale, and (F) Unified Parkinson’s Disease Rating Scale part III (UPDRS-III) score.
With regard to age, a total of 5,134 PD patients, including 2,292 PDRBD patients and 2,842 Non-RBD patients, from 30 studies were analysed. The study by Boot et al.22 was not included because the age of patients was demonstrated as a median (25th percentile, 75th percentile). Overall, elderly patients with PD were more likely to develop Confirmed-RBD than younger PD patients (SMD = 0.25; 95% CI 0.14–0.36; p = 0.000); and no significant effect of age on the occurrence of Probable-RBD (SMD = 0.09; 95% CI −0.02–0.24; p = 0.129). There was low heterogeneity across studies for analysis of Confirmed-RBD (I2 = 38.9%; p = 0.036) and no heterogeneity for Probable-RBD (I2 = 21.2%; p = 0.242) (Fig. 2B). The funnel plot did not show evidence of asymmetry (Fig. 3B).
The onset age of PD was reported in 12 studies, which included a total of 3,143 patients (1,448 PDRBD and 1,695 Non-RBD). The onset age of PD was 51.0 to 63.6 years for PDRBD patients and 48.6 to 65.5 years for Non-RBD patients. The meta-analysis showed no significant effect of onset age on the occurrence of Confirmed-RBD in PD patients (SMD = 0.08; 95% CI −0.03–0.18; p = 0.154); similarly, no significant effect of onset age on the occurrence of Probable-RBD (SMD = −0.05; 95% CI −0.23–0.14; p = 0.628). The inconsistency amongst the included studies was low (I2 = 5.3%; p = 0.386) and moderate (I2 = 54.7%; p = 0.050) respectively (Fig. 2C). No clear evidence of asymmetry was demonstrated in the funnel plot (Fig. 3C).
Twenty-eight studies reported a difference in disease duration between PDRBD and Non-RBD patients. A total of 2,623 PDRBD patients (mean disease duration from 4.8 to 15.3 years) and 2,111 Non-RBD patients (mean disease duration from 4.3 to 13.9 years) were included in the meta-analysis. A longer disease duration increased the risk of both of Confirmed-RBD and Probable-RBD in PD patients (SMD = 0.30; 95% CI 0.22–0.37; p = 0.000 and SMD = 0.29; 95% CI 0.19–0.39; p = 0.000). There was no significant inconsistency across the studies (I2 = 0.0%; p = 0.757 and 0.0%; p = 0.948 respectively) (Fig. 2D). The funnel plot did not suggest asymmetry (Fig. 3D).
Sixteen studies reported a difference in the Hoehn-Yahr score between PDRBD (n = 986) and Non-RBD patients (n = 1,496). The combined effect size was low but statistically significant for both of Confirmed-RBD and Probable-RBD (SMD = 0.30; 95% CI 0.19–0.40; p = 0.000 and SMD = 0.30; 95% CI 0.19–0.44; p = 0.000). There was no inconsistency across the studies (I2 = 0.0%; p = 0.642 and I2 = 0.0%; p = 0.564 respectively) (Fig. 2E), and the funnel plot did not suggest asymmetry (Fig. 3E).
Twenty-two studies described the UPDRS-III score for PDRBD patients (n = 1,162; mean score 1.19–38.7) and Non-RBD patients (n = 1,836; mean score 1.1–30.7). The UPDRS-III score was higher in Confirmed-RBD and Probable-RBD patients compared with Non-RBD patients (SMD = 0.38; 95% CI 0.14–0.61; p = 0.002 and SMD = 0.26; 95% CI 0.11–0.41; p = 0.001). True heterogeneity across studies was high for Confirmed-RBD (I2 = 78.9%; p = 0.0) and none for Probable-RBD (I2 = 15.0%; p = 0.315) (Fig. 2F). Two conspicuous studies8,32 that were out of range were revealed in the funnel plot (Fig. 2F). Removing these studies yielded a significant effect of the UPDRS-III score on the occurrence of RBD (SMD = 0.29; 95% CI 0.20–0.38; p = 0.000); however, heterogeneity was not observed across the studies (I2 = 17.7%; p = 0.221). Notably, UPDRS-III test performed on the state of “on”, “off” and unknown was reported in 38,40,43, 48,30,40,44 and 171,7,20,23,24,25,26,27,28,31,32,34,35,39,41,42,46 of the original studies respectively, which is inappropriate for the further sub-group analysis in this meta-analysis. Therefore, the findings concerning the connection between UPDRS-III score and the occurrence of RBD should be interpreted more cautiously.
Discussion
In the last decade, numerous studies evaluated the clinical factors associated with the occurrence of RBD in PD patients. However, the results are varied due to the diverse criteria for a diagnosis of RBD, different data sources and insufficient sample sizes in most studies. Thus, the relationship between RBD and PD remains unclear.
To summarize these previous studies and obtain a more comprehensive conclusion, we performed a meta-analysis of 33 studies with a total of 5,919 subjects who were diagnosed with PD, including 2,411 PDRBD patients. To our knowledge, this is the first meta-analysis that systematically estimated the clinical factors related to RBD in PD patients. Our findings demonstrated that Confirmed-RBD patients showed the following characteristics compared to PD patients with normal REM sleep behaviour: male, older, longer disease duration, higher disease activity (Hoehn-Yahr scale) and higher motor examination (UPDRS-III) score; while the Probable-RBD patients demonstrated the different clinical features compared to RBD patients in disease duration, disease activity and motor examination.
Previous studies suggested that the incidence of PD is greater in men than women due to the neuroprotective effects of oestrogen via stimulation of dopamine neurotransmission and greater levodopa bioavailability47,48 and/or the variant gene expression patterns in dopamine (DA) neurons in the substantia nigra pars compacta (SNc) between males and females49. Similar to previous reports, our meta-analysis demonstrated that male PD patients had a higher risk for RBD.
Evidence suggests that the pathophysiology of sleep disturbances in PD includes degenerative changes and structural lesions in brainstem areas, particularly the dorsal midbrain and pons, that are related to sleep-wake activity and sleep regulation14. With regards to clinical features, RBD was reported to be associated with older age, longer PD duration, higher disease severity expressed by Hoehn-Yahr scale, higher UPDRS motor and non-motor symptom scores, more severe motor fluctuation, and higher levodopa dosage for antiparkinsonian treatment, but there was no difference in onset age in PD patients when compared with Non-RBD patients8,41,50,51. These findings suggest that RBD in PD represents a more severe neurodegenerative process when compared with non-RBD.
At the time, our meta-analysis was conducted using all of the available literature sources that compared the clinical profiles of PD patients with and without RBD. Therefore, we consider these results to be comprehensive and valid. However, some limitations in our analysis and in the individual studies should be considered when interpreting our findings.
Previous studies revealed that antidepressants can produce dream-enactment behavior, reduce REM sleep atonia, and finally precipitate or aggravate RBD22,52,53. Even though the details for the mechanisms are unclear, the treatment of antidepressants especially selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors have been implicated in the occurrence of RBD in patients with PD22. However, in this meta-analysis, we cannot distinguish primary RBD from RBD linked to antidepressants since the data were insufficient in the original articles. Besides, in several of the included studies1,2,20,22,24,25,28,29,30,31,33,36,37,38,40,44,45, RBD was diagnosed based on a questionnaire or clinical manifestations but not confirmed with PSG. PSG can detect muscle activation patterns during REM sleep, including aberrant chin muscle tone, limb jerking, and hyperkinetic or violent behaviour; however, this technique is not part of the mandatory criteria for a clinical diagnosis2,54. Regardless, PSG is considered the “gold standard” because it provides objective parameters of primary sleep behaviour in PD patients55. Thus, a lack of PSG confirmation for an RBD diagnosis might have led to a biased prevalence of RBD in PD patients in our meta-analysis.
Heterogeneity amongst the individual studies is inevitable in meta-analyses because of variability in design characteristics and the relatively poor quality of reporting in primary studies. In this study, several high-quality studies were removed due to data that were deemed inappropriate for our meta-analysis, i.e., data that would increase the heterogeneity and weaken the stringency of the meta-analysis. The deficiencies in the data prevented the inclusion of clinical symptoms (wearing-off/fluctuations, dyskinesia, hallucinations, restless legs syndrome, hypertension, or constipation), psychological assessment parameters (Beck Depression Inventory, BDI and Mini Mental State Examination, MMSE), and treatment (e.g., Levodopa dosage and course duration) in the meta-analysis.
In summary, our data suggest that the clinical characteristics between PD patients with and without RBD are distinct, indicating that PDRBD might represent a variant pattern of neurodegeneration in PD patients. However, the limitations of our study require a cautious interpretation of the identified risk factors for RBD in PD patients. Additional investigations with a more comprehensive design are needed to determine the distinct features of PDRBD patients.
Additional Information
How to cite this article: Zhu, R.-l. et al. Clinical variations in Parkinson’s disease patients with or without REM sleep behaviour disorder: a meta-analysis. Sci. Rep. 7, 40779; doi: 10.1038/srep40779 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Program, Grant No. 2015CB856405) and National Natural Science Foundation of China (Grant No. 31300925).
Footnotes
Author Contributions Ruo-lin Zhu and Cheng-juan Xie collected and analysed the data and drafted the manuscript; Pan-pan Hu collected the data; Kai Wang provided analytical oversight and revised the manuscript. All authors have read and approved the final version of the submitted manuscript.
References
- Hu Y. et al. Parkinson disease with REM sleep behavior disorder: features, alpha-synuclein, and inflammation. Neurology. 84, 888–894 (2015). [DOI] [PubMed] [Google Scholar]
- Fantini M. L. et al. Increased risk of impulse control symptoms in Parkinson’s disease with REM sleep behaviour disorder. J Neurol Neurosurg Psychiatry. 86, 174–179 (2015). [DOI] [PubMed] [Google Scholar]
- Chiu H. F. et al. Sleep-related injury in the elderly–an epidemiological study in Hong Kong. Sleep. 23, 513–517 (2000). [PubMed] [Google Scholar]
- Poryazova R., Oberholzer M., Baumann C. R. & Bassetti C. L. REM Sleep Behavior Disorder in Parkinson’s Disease: A Questionnaire-Based Survey. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine. 9, 55–59 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comella C. L., Nardine T. M., Diederich N. J. & Stebbins G. T. Sleep-related violence, injury, and REM sleep behavior disorder in Parkinson’s disease. Neurology. 51, 526–529 (1998). [DOI] [PubMed] [Google Scholar]
- Postuma R., Gagnon J. F., Vendette M., Charland K. & Montplaisir J. REM sleep behaviour disorder in Parkinson’s disease is associated with specific motor features. J Neurol Neurosurg Psychiatry. 79, 1117–1121 (2008). [DOI] [PubMed] [Google Scholar]
- Suzuki K. et al. Probable rapid eye movement sleep behavior disorder, nocturnal disturbances and quality of life in patients with Parkinson’s disease: a case-controlled study using the rapid eye movement sleep behavior disorder screening questionnaire. BMC neurology. 13, 1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. E. et al. Rapid eye movement sleep behavior disorder after bilateral subthalamic stimulation in Parkinson’s disease. J Clin Neurosci. 22, 315–319 (2015). [DOI] [PubMed] [Google Scholar]
- Schenck C. H., Bundlie S. R., Ettinger M. G. & Mahowald M. W. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 9, 293–308 (1986). [DOI] [PubMed] [Google Scholar]
- Schenck C. H., Bundlie S. R. & Mahowald M. W. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder. Neurology. 46, 388–393 (1996). [DOI] [PubMed] [Google Scholar]
- Iranzo A. et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. 5, 572–577 (2006). [DOI] [PubMed] [Google Scholar]
- Spillantini M. G. & Goedert M. The α‐Synucleinopathies: Parkinson’s Disease, Dementia with Lewy Bodies, and Multiple System Atrophy. Ann N Y Acad Sci. 920, 16–27 (2000). [DOI] [PubMed] [Google Scholar]
- Postuma R. et al. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology. 72, 1296–1300 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeve B. F. et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 130, 2770–2788 (2007). [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J. & Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj. 339, b2535 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadad A. R. et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 17, 1–12 (1996). [DOI] [PubMed] [Google Scholar]
- Bowden J., Tierney J. F., Copas A. J. & Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med Res Methodol. 11, 41 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P. & Thompson S. G. Quantifying heterogeneity in a meta-analysis. Stat Med. 21, 1539–1558 (2002). [DOI] [PubMed] [Google Scholar]
- Yin Y., Wu X., Shan G. & Zhang X. Diagnostic value of serum anti-C1q antibodies in patients with lupus nephritis: a meta-analysis. Lupus. 21, 1088–1097 (2012). [DOI] [PubMed] [Google Scholar]
- Arnaldi D. et al. Functional neuroimaging and clinical features of drug naive patients with de novo Parkinson’s disease and probable RBD. Parkinsonism Relat Disord (2016). [DOI] [PubMed] [Google Scholar]
- Arnulf I. et al. Sleepiness in idiopathic REM sleep behavior disorder and Parkinson disease. Sleep. 38, 1529 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boot B. P. et al. Probable rapid eye movement sleep behavior disorder increases risk for mild cognitive impairment and Parkinson disease: a population-based study. Ann Neurol. 71, 49–56 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fabio N., Poryazova R., Oberholzer M., Baumann C. R. & Bassetti C. L. Sleepwalking, REM sleep behaviour disorder and overlap parasomnia in patients with Parkinson’s disease. Eur Neurol. 70, 297–303 (2013). [DOI] [PubMed] [Google Scholar]
- Ford A. H. et al. Rapid eye movement sleep behavior disorder in Parkinson’s disease: magnetic resonance imaging study. Mov Disord. 28, 832–836 (2013). [DOI] [PubMed] [Google Scholar]
- Gjerstad M. D., Boeve B., Wentzel-Larsen T., Aarsland D. & Larsen J. Occurrence and clinical correlates of REM sleep behaviour disorder in patients with Parkinson’s disease over time. Journal of Neurology, Neurosurgery & Psychiatry. 79, 387–391 (2008). [DOI] [PubMed] [Google Scholar]
- Gong Y. et al. Clinical manifestations of Parkinson disease and the onset of rapid eye movement sleep behavior disorder. Sleep medicine. 15, 647–653 (2014). [DOI] [PubMed] [Google Scholar]
- Janković M., Svetel M. & Kostić V. Frequency of REM sleep behavior disorders in patients with Parkinson’s disease. Vojnosanitetski pregled. 72, 442–446 (2015). [DOI] [PubMed] [Google Scholar]
- Kim C. S., Sung Y. H., Kang M. J. & Park K. H. Rapid Eye Movement Sleep Behavior Disorder in Parkinson’s Disease: A Preliminary Study. J Mov Disord. 9, 114 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. E. et al. REM sleep behavior disorder: association with motor complications and impulse control disorders in Parkinson’s disease. Parkinsonism Relat Disord. 20, 1081–1084 (2014). [DOI] [PubMed] [Google Scholar]
- Kotagal V. et al. Symptoms of rapid eye movement sleep behavior disorder are associated with cholinergic denervation in Parkinson disease. Annals of neurology. 71, 560–568 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. E., Kim K. S., Shin H.-W. & Sohn Y. H. Factors related to clinically probable REM sleep behavior disorder in Parkinson disease. Parkinsonism Relat Disord. 16, 105–108 (2010). [DOI] [PubMed] [Google Scholar]
- Lim J. S. et al. Neural substrates of rapid eye movement sleep behavior disorder in Parkinson’s disease. Parkinsonism Relat Disord. 23, 31–36 (2016). [DOI] [PubMed] [Google Scholar]
- Mahale R., Yadav R. & Pal P. Rapid eye movement sleep behaviour disorder in young-and older-onset Parkinson disease: a questionnaire-based study. Sleep Med. 15, 642–646 (2014). [DOI] [PubMed] [Google Scholar]
- Neikrug A. B. et al. Parkinson’s disease and REM sleep behavior disorder result in increased non-motor symptoms. Sleep Med. 15, 959–966 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nihei Y. et al. REM sleep behavior disorder in Japanese patients with Parkinson’s disease: a multicenter study using the REM sleep behavior disorder screening questionnaire. J Neurol. 259, 1606–1612 (2012). [DOI] [PubMed] [Google Scholar]
- Nomura T., Inoue Y., Kagimura T. & Nakashima K. Clinical significance of REM sleep behavior disorder in Parkinson’s disease. Sleep Med. 14, 131–135 (2013). [DOI] [PubMed] [Google Scholar]
- Özekmekçi S., Apaydin H. & Kiliç E. Clinical features of 35 patients with Parkinson’s disease displaying REM behavior disorder. Clinical neurology and neurosurgery. 107, 306–309 (2005). [DOI] [PubMed] [Google Scholar]
- Poryazova R., Oberholzer M., Baumann C. R. & Bassetti C. L. REM sleep behavior disorder in Parkinson’s disease: a questionnaire-based survey. J Clin Sleep Med. 9, 55–59A (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma R. B. et al. Rapid eye movement sleep behavior disorder and risk of dementia in Parkinson’s disease: a prospective study. Mov Disord. 27, 720–726 (2012). [DOI] [PubMed] [Google Scholar]
- Sinforiani E. et al. REM sleep behavior disorder, hallucinations, and cognitive impairment in Parkinson’s disease. Mov Disord. 21, 462–466 (2006). [DOI] [PubMed] [Google Scholar]
- Sixel-Döring F., Trautmann E., Mollenhauer B. & Trenkwalder C. Associated factors for REM sleep behavior disorder in Parkinson disease. Neurology. 77, 1048–1054 (2011). [DOI] [PubMed] [Google Scholar]
- Sorensen G. L., Mehlsen J. & Jennum P. Reduced sympathetic activity in idiopathic rapid-eye-movement sleep behavior disorder and Parkinson’s disease. Auton Neurosci. 179, 138–141 (2013). [DOI] [PubMed] [Google Scholar]
- Vendette M. et al. REM sleep behavior disorder predicts cognitive impairment in Parkinson disease without dementia. Neurology. 69, 1843–1849 (2007). [DOI] [PubMed] [Google Scholar]
- Vibha D. et al. RBD in Parkinson’s disease: A clinical case control study from North India. Clin Neurol Neurosurg. 113, 472–476 (2011). [DOI] [PubMed] [Google Scholar]
- Yoritaka A., Ohizumi H., Tanaka S. & Hattori N. Parkinson’s disease with and without REM sleep behaviour disorder: are there any clinical differences? European neurology. 61, 164–170 (2009). [DOI] [PubMed] [Google Scholar]
- Zhang L.-Y. et al. Association of rapid eye movement sleep behavior disorder with sleep-disordered breathing in Parkinson’s disease. Sleep Medicine. 20, 110–115 (2016). [DOI] [PubMed] [Google Scholar]
- Shulman L. M. Gender differences in Parkinson’s disease. Gender medicine. 4, 8–18 (2007). [DOI] [PubMed] [Google Scholar]
- Van Den Eeden S. K. et al. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 157, 1015–1022 (2003). [DOI] [PubMed] [Google Scholar]
- Cantuti-Castelvetri I. et al. Effects of gender on nigral gene expression and parkinson disease. Neurobiol Dis. 26, 606–614 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnulf I. REM sleep behavior disorder: motor manifestations and pathophysiology. Mov Disord. 27, 677–689 (2012). [DOI] [PubMed] [Google Scholar]
- Postuma R., Gagnon J., Vendette M. & Montplaisir J. Markers of neurodegeneration in idiopathic rapid eye movement sleep behaviour disorder and Parkinson’s disease. Brain. 132, 3298–3307 (2009). [DOI] [PubMed] [Google Scholar]
- Winkelman J. W. & James L. Serotonergic antidepressants are associated with REM sleep without atonia. Sleep. 27, 317–321 (2004). [DOI] [PubMed] [Google Scholar]
- Postuma R. B. et al. Antidepressants and REM sleep behavior disorder: isolated side effect or neurodegenerative signal? Sleep. 36, 1579–1585 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre O. & Montplaisir J. Polysomnographic features of REM sleep behavior disorder Development of a scoring method. Neurology. 42, 1371–1371 (1992). [DOI] [PubMed] [Google Scholar]
- Peeraully T., Yong M. H., Chokroverty S. & Tan E. K. Sleep and Parkinson’s disease: A review of case‐control polysomnography studies. Mov Disord. 27, 1729–1737 (2012). [DOI] [PubMed] [Google Scholar]