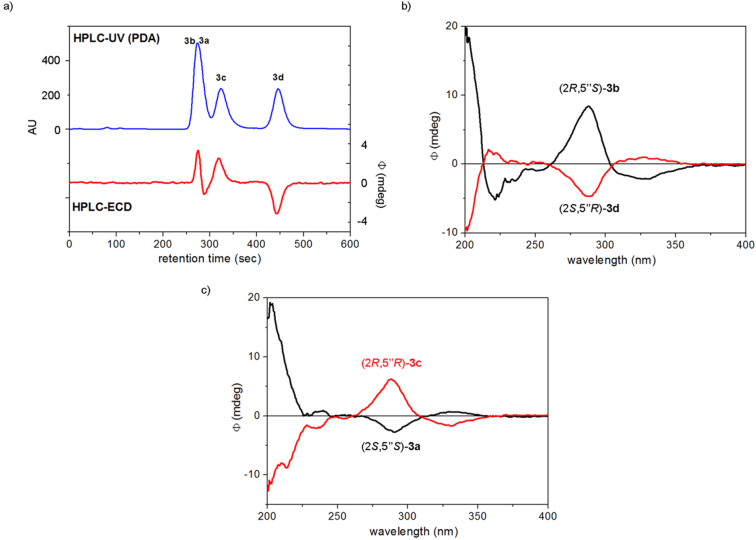
Figure 9.
a) Chiral HPLC–UV and HPLC–ECD traces of dracocephins B1–B4 3a–d using a Chiralpak IC column with the eluent MeCN/2-propanol/TFA 90:10:0.1 monitored at 285 nm. b) HPLC–ECD spectra of the first- [black: (2R,5”S)-3b or dracocephin B2] and fourth eluted [red: (2S,5”R)-3d or dracocephin B4] stereoisomers of dracocephins B. c) HPLC–ECD spectra of the second [black: (2S,5”S)-3a or dracocephin B1] and third eluted [red: (2R,5”R)-3c or dracocephin B3] stereoisomers of dracocephins B. The absolute configurations were assigned on the basis of the publication of Ren et al. [2].