Table 3.
Syntheses of 3,5-disubstituted hydantoins under dry-grinding (conditions A)a or PEG-assisted grinding (conditions B and C).b
| Entry | H-AA-OMe | Yields (%)b vs conditionsa | Product | ||
| A | B | C | |||
| 1 | H-Leu-OMe | 61 [9] | 70 | 73 |
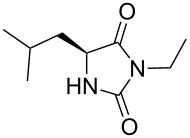 2a [9] |
| 2 | 57 [9] | 69 | 66c |
 2b [9] |
|
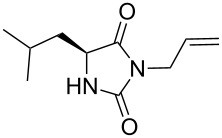
| 3 | 38 [9] | n.p.d | 48c |
 2c [9] |
|
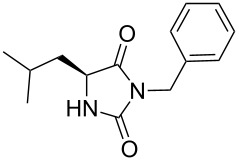
| 4 | H-Phe-OMe | 84 [9] | 70 | 60 |
 3a [9] |
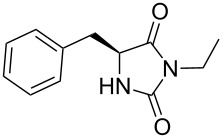
| 5 | 30 | n.d.e | n.d.e |
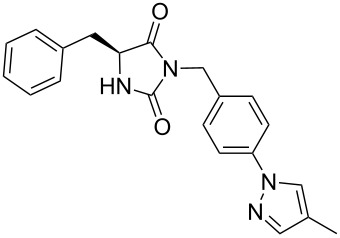 3b |
|
| 6 | 70 | n.d.e | n.d.e |
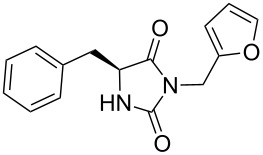 3c |
|
| 7 | H-Ser(Ot-Bu)-OMe | 51 [9] | 70 | 70 |
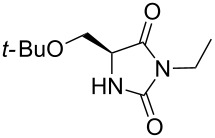 4 |
| 8 | H-Lys(Z)-OMe | 31 [9] | 47c | 50c |
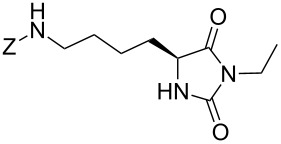 5a [9] |
| 9 | 62 | n.p.d | 37c |
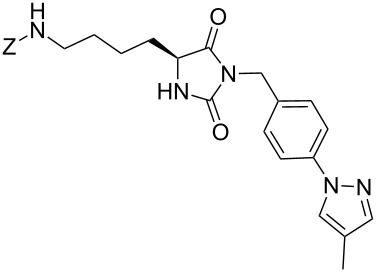 5b |
|
| 10 | 37 | n.p.d | n.p.d |
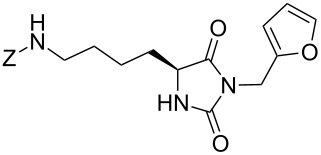 5c |
|
| 11 | 47 | n.p.d | n.p.d |
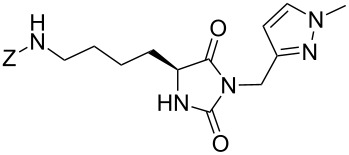 5d |
|
| 12 | H-Aib-OMe | 46 [9] | 62 | 62 |
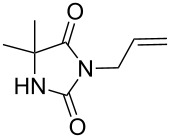 6 [9] |
aConditions: (step 1) (L)-α-amino ester hydrochloride (1 equiv) and CDI (1.3 equiv) at 450 rpm, in a 12 mL inox jar with 50 balls (stainless steel, 5 mm Ø) for 40 min; (step 2) R2NH2 (1.6 equiv) and K2CO3 (3.6 equiv) at 450 rpm for 2 hours. A: the reaction was performed with no additive (dry-grinding); B: MeO-PEG-2000-OMe (450 mg mmol−1); C: HO-PEG-3400-OH (450 mg mmol−1) were added in the second step (wet-grinding conditions with a PEG additive; bisolated yields; c1H NMR yield on the crude reaction mixture; dthe reaction was not performed (n.p.); ethe reaction yield was not determined (n.d.).
