Abstract
Sodium dynamics are essential for regulating functional processes in glial cells. Indeed, glial Na+ signaling influences and regulates important glial activities, and plays a role in neuron-glia interaction under physiological conditions or in response to injury of the central nervous system (CNS). Emerging studies indicate that Na+ pumps and Na+-dependent ion transporters in astrocytes, microglia, and oligodendrocytes regulate Na+ homeostasis and play a fundamental role in modulating glial activities in neurological diseases. In this review, we first briefly introduced the emerging roles of each glial cell type in the pathophysiology of cerebral ischemia, Alzheimer’s disease, epilepsy, Parkinson’s disease, Amyotrophic Lateral Sclerosis, and myelin diseases. Then, we discussed the current knowledge on the main roles played by the different glial Na+-dependent ion transporters, including Na+/K+ ATPase, Na+/Ca2+ exchangers, Na+/H+ exchangers, Na+-K+-Cl− cotransporters, and Na+-HCO3− cotransporter in the pathophysiology of the diverse CNS diseases. We highlighted their contributions in cell survival, synaptic pathology, gliotransmission, pH homeostasis, and their role in glial activation, migration, gliosis, inflammation, and tissue repair processes. Therefore, this review summarizes the foundation work for targeting Na+-dependent ion transporters in glia as a novel strategy to control important glial activities associated with Na+ dynamics in different neurological disorders.
Keywords: astrocytes, microglia, oligodendrocytes, Na+/Ca2+ exchanger, Na+/H+ exchanger, Na+/K+ ATPase, Na+-HCO3− cotransporter, Na+-K+-Cl− cotransporter
Introduction
Sodium (Na+) dynamics are essential for regulating important glial activities and play a role in neuron–glia interaction under physiological conditions and in response to CNS injury (Fields, 2015; Rose and Karus, 2013; Zuchero and Barres, 2015). The amplitude and spatial distribution of intracellular Na+ transients in glial cells is governed by the cellular distribution of the diverse Na+ influx and efflux pathways. Among them, an increasing number of evidence indicates that many Na+-dependent ion transporters in glial cells are responsible for the generation of relevant glial Na+ signals during pathophysiological conditions, with significant functional consequences on cell survival, gliotransmission, pH homeostasis, as well as on glial activation, migration, gliosis, inflammation, and tissue repair processes. Glial intracellular Na+ homeostasis is regulated by the concerted activities of ion transporters including the Na+/K+ ATPase (NKA), Na+/Ca2+ exchangers (NCX), the Na+-K+-Cl− cotransporter isoform 1 (NKCC1), Na+/H+ exchangers (NHE), and Na+-HCO3− cotransporters (NBC) (Annunziato et al., 2013; Rose and Verkhratsky, 2016). Altered function of these ion transporters has been detected in experimental models of various disorders of the CNS (Figs. 1 and 2).
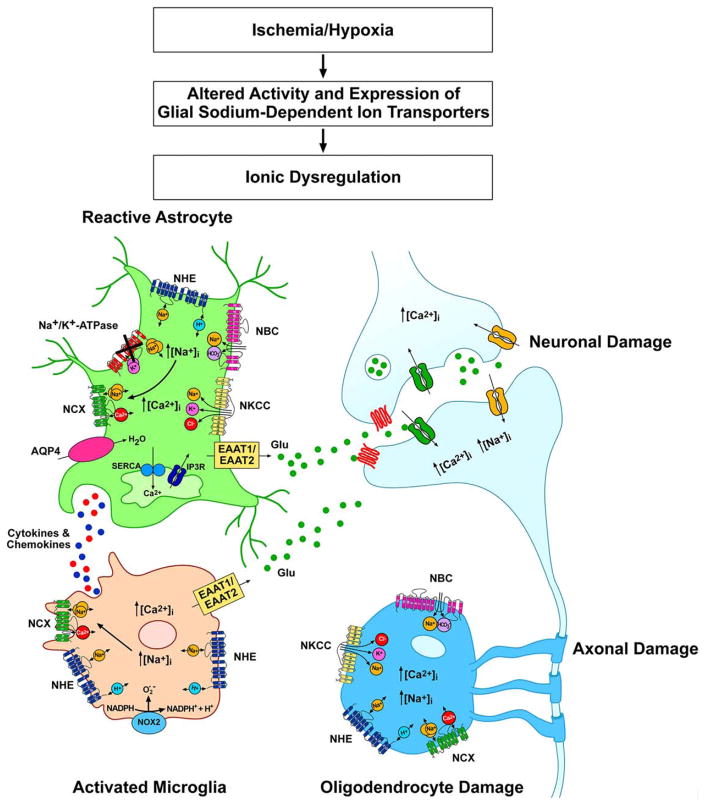
FIGURE 1.
Schematic representation of the Na+-dependent transporters involved in Na+ dynamics during the hypoxic-ischemic insult in astrocytes, microglia, and oligodendrocytes.
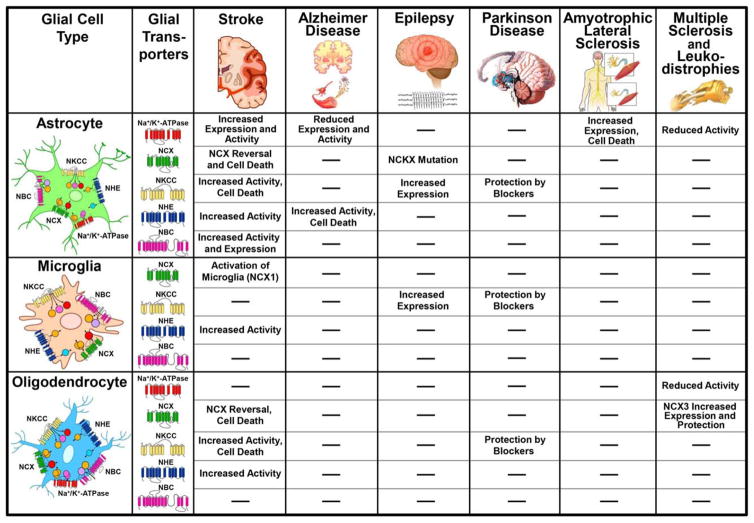
FIGURE 2.
Schematic representation of the emerging roles of the glial Na+-dependent transporters in different neurological disorders. According to the different CNS diseases the diagram summarizes the major alterations in expression level and activity for each Na+ dependent transporter in astrocytes, microglia and oligodendrocytes. Similarly, the contribution of each Na+ dependent transporter to cell survival/death, and diverse glial activities is also indicated. The symbol (−) indicates “not directly tested.”
This review collectively summarizes new findings on these well studied Na+-dependent ion transporters in glial function under pathophysiological conditions. These findings demonstrate the potentials of these proteins as therapeutic targets for neurological diseases. The role of other Na+ ion channels and ion transporters in glial function have also been discussed in other reviews (Kirischuk et al., 2012; Majumdar and Bevensee, 2010; Pappalardo et al., 2016).
Stroke
The ischemic insult may trigger glial cell death in the ischemic core, but surviving astrocytes, microglia, and oligodendrocytes in the peri-ischemic region produce an extensive response in the post-ischemic brain that may play either beneficial or detrimental roles on restoring brain functions after injury (Kettenmann et al., 2011).
Astrocytes are the most ischemia-resistant glial elements, as they are able to survive in conditions of limited blood supply and may protect brain against injury. In fact, they are able to sustain anaerobic metabolism in hypoxic condition, act as a powerful scavengers of reactive oxygen species (ROS), remove the excess of potassium released during ischemia, and slow down the neuronal depolarization and depolarization-induced glutamate release (Wilson, 1997; Rose and Karus, 2013; Verkhraski et al., 2013). In stroke, astrocytes are also involved in the regulation of water and ion homeostasis, cerebral blood flow, maintenance of the blood-brain barrier, and in the control of the extracellular levels of glutamate, as well as being a source of a number of neuroprotective factors. In contrast, reactive astrocytes may exacerbate the cell damage resulting from their participation in inflammatory processes and production of neurotoxic mediators (Rose and Karus, 2013). Astrocytes respond to the ischemic insult by developing reactive astrogliosis. Although the general view is that astrocytic scars are critical inhibitors of the CNS axon regeneration (Davies et al., 1997), Anderson et al. (2016) recently reported that scar-forming astrocytes strongly support axon regeneration. Therefore, reactive astrocytes could present protective and harmful phenotypes, which are associated with beneficial or detrimental forms of gliosis (Khakh and Sofroniew, 2015). Understanding the signals that drive astrocytes towards neuroprotective phenotypes will help to attenuate damage following brain injury.
Microglia activation characterizes the first inflammatory response to ischemic injury. Microglia undergo morphological transformation from resting into activated and phagocytic state and migrate to the site of injury (Annunziato et al., 2013; Kettenmann et al., 2011). Polarization of activated microglia into distinct phenotypes may explain their roles in inflammation and tissue repair function in the post-ischemic brain (Hu et al., 2015).
Oligodendrocytes, the myelin-forming cells of the CNS, are the most vulnerable and sensitive glial cells to ischemia. Oligodendrocyte pathology results in demyelination which has profound consequences for axonal function and neuronal survival (Pantoni et al., 1996). Oligodendrogenesis participate to the repair processes after stroke (Zhang et al., 2013). In fact, adult oligodendrocyte precursor cells (OPC) resident in the white matter or derived from neural progenitor cells in the subventricular zone contribute to the generation of mature myelinating oligodendrocytes, and are capable to remyelinate demyelinated axons, restore the saltatory conduction and improve motor and sensory function after stroke (Li et al., 2010; Nave, 2010). A deeper understanding of the ionic mechanisms controlling oligodendrogenesis in the adult brain is warranted to develop novel therapeutic strategies to enhance repair processes and reduce neurological deficits after stroke.
NCX
NCX is a major player in the regulation of intracellular sodium and calcium dynamics under phatological conditions. Depending on the concentrations of these two ions and on the plasma membrane potential the antiporter may operate via the forward mode, also named Na+ influx pathway, or reverse mode, also named Na+ efflux pathway (Annunziato et al., 2004, 2007a, 2007b). The importance of NCX function in the ischemic brain is revealed by the findings showing that the specific knocking down of the ncx1 gene as well as ncx2 and ncx3 increases the extent of the ischemic lesion in rats and mice (Jeon et al., 2008; Molinaro et al., 2008, 2015; Pignataro et al., 2004; Scorziello et al., 2013; Secondo et al., 2007, 2015). Initial studies in in vivo models of ischemia indicated that NCX isoforms display a different expression pattern in brain cells of the ischemic core and peri-infarct regions (Boscia et al., 2006, 2013, 2016), suggesting a differential contribution of each neuronal and glial NCX isoform in response to the insult.
Astrocytes
All three NCX isoforms are expressed in astrocytes where they are enriched at distal processes surrounding synapses (Minelli et al., 2007; Papa et al., 2003). Among the three NCX isoforms, NCX1 transcripts are the most highly represented in astrocytes (Pappalardo et al., 2014).
Recent work indicated the importance of [Na+]i dynamics in astroglial excitability and cellular homeostasis, with a prominent mechanism involving the transmembrane movements of Na+ and Ca2+ through NCX (Kirischuk et al., 2012; Parpura and Verkhratsky, 2012; Rose and Karus, 2013). In fact, Na+ signals in astrocytes influence cellular Ca2+ signaling through NCX. Reyes et al., (2012) reported that in cultured cortical astrocytes, NCX can operate in the reverse mode even in resting conditions and can dynamically switch between the two modes of operation in response to small increase in [Na+]i and/or depolarization. This is because the reversal potential of NCX in astrocytes is set at levels close to the resting membrane potential (Kirischuk et al., 1997; Paluzzi et al., 2007; Reyes et al., 2012). The reverse mode of NCX has been implicated in astrocyte cell death following oxygen and glucose deprivation (OGD) plus reoxygenation or exogenous glutamate application (Floyd et al., 2005). In fact, the largest Na+ increase following in vitro ischemia is observed during the reoxygenation phase due to reactivation of NKCC1 and NHE (Rose et al., 1998; Lenart et al., 2004; Kintner et al., 2007). This Na+ load causes reversal of NCX and cellular Ca2+ influx eventually resulting in mitochondrial Ca2+ accumulation. This is regarded as a main trigger for the opening of the mitochondrial permeability transition pore and cell death (Bondarenko et al., 2014; Kintner et al., 2007; Lenart et al., 2004). In accordance, the blockade of reverse NCX activity with KB-R7943 (Matsuda et al., 2001) or SEA0400 (Liu et al., 2010) attenuates injury of cultured astrocytes and OGD/reoxygenation-mediated cytocrome c release.
NCX may also contribute to the astrocyte response to the injury and to gliotransmitter release. Mechanical stimulation of cultured astroglia, which is an in vitro model to study astrocyte activation and astrogliosis, promotes a prolonged increase in [Na+]i and leads to reversal of NCX (Floyd et al., 2005; Reyes et al., 2012). More recently, Pappalardo et al. (2014) found that reversal of NCX, triggered by increased Na+ influx through Nav1.5 channel activity, plays an important role in Ca2+-dependent astrocyte migration, proliferation, and activation of gene transcription (Berridge, 1995; Gao et al., 2013). Furthermore, Ca2+ entry through NCX appears important for the elevation of cytoplasmic [Ca2+]i necessary for mechanically induced Ca2+-dependent exocytotic release of glutamate from astrocytes (Reyes et al., 2012).
Microglia
Among the three NCX genes, NCX1 is the isoform most highly expressed in microglia (Newell et al., 2007; Quednau et al., 1997). The importance of NCX1 in microglial activities is provided by the findings showing that ncx1−/− embryos have no detectable microglia in the brain (Ginhoux et al., 2010). Studies performed ex vivo, in microglia isolated from the ischemic core, and in vitro, in cultured microglia, suggest that a Na+-dependent Ca2+ influx through NCX operating in the reverse mode is necessary for microglial activation under ischemic conditions (Boscia et al., 2009; Hoffmann et al., 2003; Liu et al., 2010; Newell et al., 2007) and for bradykinin-induced microglial motility (Ifuku et al., 2007).
NCX1 immunoreactive signal was found to be progressively increased in round phagocytic microglia/macrophages invading the infarct core 3 and 7 days after pMCAO (Boscia et al., 2009). In vitro studies demonstrated that the direct exposure of cultured microglia to interferon-γ or nitric oxide, which can be released under pathophysiological conditions such as cerebral ischemia, enhanced NCX1 transcript levels (Matsuda et al., 2006; Nagano et al., 2004). NCX1 expression was significantly upregulated in BV2 microglia exposed to OGD followed by reoxygenation (Boscia et al., 2009). In these cells, NCX1 silencing completely prevented the intracellular Ca2+ rise triggered by the hypoxic-ischemic insult during the reoxygenation phase, further demonstrating that enhanced NCX1, operating in the reverse mode, is responsible for the increase in [Ca2+]i observed following OGD-Reoxygenation in microglia. More recently, Liu et al. (2010) demonstrated a concerted action of NHE1 and NCX in regulating the activation of microglia following OGD/Reoxygenation. For instance, NHE1-mediated Na+ overload may be in part responsible for the activation of NCX in the reverse mode of operation. This aspect is discussed in more detail in “NHE” section.
Oligodendrocytes
Expression and functional studies suggest that NCX1 is the main contributor to NCX currents recorded in OPC (Boscia et al., 2012; Tong et al., 2009), while NCX3 expression and activity are strongly upregulated during OPC maturation and is the main isoform expressed in mature oligodendrocytes (Boscia et al., 2012).
The reverse-mode operation of NCX in conjunction with NKCC1 stimulation has been implicated in oligodendrocyte cell death induced by AMPA-mediated excitotoxicity. Activation of AMPA receptors leads to NKCC1 phosphorylation that, in turn, enhances NKCC1-mediated Na+ influx. The latter triggers NCX in the reverse mode of operation with consequent Ca2+ overload, thereby compromising mitochondrial function and cellular viability (Chen et al., 2007).
The role of NCX in oligodendrocyte response in the post-ischemic brain is still unknown. However, based on the recent findings showing that NCX3 contributes to oligodendrocyte response following experimental autoimmune encephalomyelitis (EAE)- induced demyelination (Casamassa et al., 2016), it is plausible to hypothesize that intracellular Na+ and Ca2+ signaling through NCX3 exchanger may also be implicated in oligodendrocyte response after stroke-induced demyelination.
NHE
Astrocytes
NHE is the most important plasmamembrane transporter responsible not only for pH regulation but also for the maintenance of Na+ levels in astrocytes (Leng et al., 2014; Luo and Sun, 2007; Orlowski and Grinstein, 2004; Pedersen et al., 2006; Zhao et al., 2016).
Studies indicate that intracellular acidosis is the major stimulus that regulates NHE1 activity in both neurons and glial cells (Yao et al., 1999; Kintner et al., 2004). As the pHi gets more acidic, a rapid increase in NHE1 activity occurs reaching a maximum velocity in approximately one pH unit (Hill coefficient >1) and thereby minimizes excess cytoplasmic acidification (Aronson, 1985; Paris and Pouysstgur, 1984). An increased pHi recovery rate was detected in astrocytes subjected to in vitro ischemia (OGD/Reoxygenation) (Kintner et al., 2005).
Activation of NHE1 is also mediated through phosphorylation by protein kinases like, protein kinase A (PKA), protein kinase C (PKC), extracellular signal regulated kinases (ERK1/2) and p90(RSK) kinases, Rho activated kinase p160ROCK, and Ca2+/calmodulin dependent kinases (Kintner et al., 2005; Maly et al., 2002; Tominaga et al., 1998; Yao et al., 2001). ERK1/2 and p90(RSK) kinases mediated activation of NHE1 in neurons and its role in neuronal damage have been detected in in vitro and in vivo stroke models (Luo and Sun, 2007; Manhas et al., 2010). Inhibition of ERK activity with PD-98059 blocked the pHi recovery in astrocytes after OGD/Reoxygenation, suggesting the involvement of ERK1/2 pathways in NHE1 activation (Kintner et al., 2005).
Overstimulation of NHE1 in neurons and astrocytes also leads to massive increase in [Na+]i (Hwang et al., 2008; Kersh et al., 2009; Luo et al., 2005; Vornov et al., 1996).
In cultured mouse cortical astrocytes, elevated NHE1 activity resulted in approximately five fold rise in [Na+]i, from a resting level of 11.3–61.2 mM after OGD/Reoxygenation (Kintner et al., 2004). Genetic ablation of astrocytic NHE1 or pharmacological inhibition of NHE1 with its inhibitor HOE-642 abolished the pHi recovery and reduced the Na+ loading by ~60% (Kintner et al., 2004). In mouse hippocampal astrocytes, 2 h OGD triggered a significant increase in NHE1 protein expression and stimulated NHE1 mediated H+ efflux (Cengiz et al., 2014). The sustained activation of NHE1 contributed to intracellular Na+ and Ca2+ overload which can be abolished by pharmacological inhibition with HOE-642. Similar findings were demonstrated in cultured cortical astrocytes when superfused with a solution that mimic the ionic composition of the ischemic extracellular space (Bondarenko and Chesler, 2001; Bondarenko et al., 2005). NHE1 stimulation caused an initial rapid decline in astrocyte pHi and a recovery of pHi overshoot of the baseline when cells were returned to the normal buffer and, HOE-642 prevented the pHi overshoot (Bondarenko et al., 2005). These findings suggest that overstimulation of NHE1 activity is paradoxically involved in correcting pHi and astrocytic Na+ overload and is detrimental to astrocytes after ischemia.
Because the reversal potential of NCX is close to the resting membrane potential in astrocytes (Vm~−80 mV) (Rose and Verkhrassky, 2016), the rise in [Na+]i can drive NCX to operate in reverse mode and cause intracellular Ca2+ overload. In mouse cortical astrocytes, potent inhibitors of reversed NCX, SEA0400, and KB-R7943, abolished both cytosolic Ca2+ increase and the Ca2+ oveload in the intracellular organelles after OGD/Reoxygenation (Kintner et al., 2005). Inhibition of ER Ca2+ ATPase with thapsigargin caused over two fold increase in Ca2+ release from ER, which was sensitive to blockade of NHE1 activity with HOE-642 or deletion of NHE1, suggesting that stimulation of NHE1 activity also affects ER Ca2+ loading following OGD/Reoxygenation (Kintner et al., 2005). Bondarenko et al. (2005) reported that KB-R7943 reduces the NHE1-mediated rise in [Ca2+]i after exposing astrocytes to hypoxic conditions. Excessive increase in [Ca2+]i promotes cell damage by activation of multiple cytotoxic mechanisms, including mitochondrial permeability transition, disruption of cytoskeletal organization, and activation of various proteases and phospholipases leading to apoptotic cell death (Bano and Nicotera, 2007; Zundorf and Reiser, 2011), which can be reduced by either pharmacological inhibition or genetic deletion of NHE1. These studies clearly illustrate that NHE1 and NCX are involved in dysregulation of intracellular Na+ and Ca2+ homeostasis and subsequent astrocyte death after ischemic injury.
Over-stimulation of NHE1 can also lead to release of glutamate via reversal of Na+-dependent glutamate transporters (Fig. 1). Na+-dependent glutamate transporters are responsible for excessive glutamate release and leads to a cytotoxic cell damage during ischemic stroke (Malarkey and Parpura, 2008). A recent study by Cengiz et al. (2014) reported that the sustained activation of NHE1 in reactive hippocampal astrocytes not only resulted in intracellular Na+ and Ca2+ overload but also caused a robust release of gliotransmitters and proinflammatory cytokines after in vitro ischemia. Blocking the activity of NHE1 increased Na+-dependent glutamate uptake and also significantly reduced the release of cytokines (Cengiz et al., 2014). Collectively, these results suggest that NHE1 activity regulates release of astrocytic cytotoxic components after ischemia.
Microglia
NHE1 is the most abundant isoform in microglia and involved in microglial activation (Liu et al., 2010). NHE1 plays a pivotal role in maintaining resting pHi (Faff et al., 1996; Liu et al., 2010) and accelerates pHi regulation in microglia. Inhibition of NHE1 with HOE-642 or EIPA not only acidified the cells but also abolished the pHi recovery (Liu et al., 2010). Similar to astrocytes, overstimulation of NHE1 activity causes an intracellular Na+ overload in microglia after OGD/Reoxygenation, from a basal level of ~12–28 mM. A twofold rise in [Ca2+]i was detected in microglia which was due to the concerted activation of NHE1 and reverse mode activation of NCX (Liu et al., 2010). Moreover, the rise of [Ca2+]i was prevented by inhibition of NHE1 activity with HOE-642 or reversed NCX with SEA0400. These studies provide first line of evidence indicating the role of NHE1 in triggering Na+ and Ca2+ signaling in microglia.
Na+-dependent Ca2+ rise is critical for microglial activation and generation of ROS during anoxia and ischemia (Brookes et al., 2004; Li et al., 2000). In primary microglia, OGD/Reoxygenation induced a ~17 fold increase in superoxide anion ( ) production and it was reduced to ~50% by HOE-642 treatment. Activation of microglia with phorbol 12-myristate 13-acetate (PMA), a potent stimulator of NOX activity, also resulted in similar increase in ( ) production which was suppressed by inhibition of NHE1 activity, implicating the role of NHE1 in NADPH oxidase activation and ( ) production (Liu et al., 2010) (Fig. 1). Liu et al., (2010) showed that the production of ( ) also resulted in increased mRNA expression of proinflammatory cytokines such as IL-1β, IL-6, TNFα, and nitric oxide synthase 2, in stimulated microglia. Similar results were also observed in ischemic brains. Blockade of NHE1 reduced NADPH oxidase activation and proinflammatory cytokine production (Shi et al., 2011), suggesting for a role of NHE1 in neuroinflammation in ischemic stroke.
In summary, both in vitro and animal experimental studies indicate that NHE1 activity in glial cells was stimulated under ischemia/hypoxia pathological conditions and contributed to overall brain damage by (a) cellular alkalinization, (b) intracellular Na+ and Ca2+ overload, (c) the release of gliotransmitters and proinflammatory cytokines (Fig. 1).
NKCC
NKCC1 is abundantly expressed in neuronal and non-neuronal cells of the cerebrum, cerebellum, spinal cord, PNS and choroid plexus, and in the cerebral vascular endothelial cells (Plotkin et al., 1997a). NKCC1 promotes the electroneutral transport of 1 Na+, 1 K+, and 2Cl− ions across the plasma membrane. Its activity plays an important role in epithelial Na+ secretion and absorption, maintenance of intracellular Na+ concentration, cell volume regulation, intracellular Cl− homeostasis and K+ uptake (Ernest and Sontheimer, 2007; Jayakumar et al., 2008; Kahle et al., 2008; Kristensen et al., 2008).
Astrocytes
The basal [Na+]i level in astrocytes is reported to be in the range of ~8–19 mM (Lenart et al., 2004; Longuemare et al., 1999; Rose and Ransom, 1996; Rose et al., 1998). An increase in [Na+]i is found in rat spinal cord astrocytes (Rose et al., 1998), rat cortical astrocytes (Longuemare et al., 1999), and mouse cortical astrocytes (Silver and Ericinska, 1997) exposed to glucose deprivation, chemical hypoxia, or ischemia. Stimulation of NKCC1 activity by OGD resulted in ~3.6 fold increase in [Na+]i in astrocytes. Blocking the NKCC1 activity either by bumetanide or genetic ablation reduced the Na+ rise by ~50%, indicating NKCC1-mediated Na+ influx (Lenart et al., 2004). NKCC1-mediated Na+ overload can also be seen upon exposure of astrocytes to hypoxic acidic ion-shifted Ringer (HAIR) solution, whose composition mimics the hypoxic/ischemic conditions. A decrease in basal [Na+]i, from ~13.5 mM to 6.6 mM, was seen in astrocytes upon 5-min exposure to HAIR solution (Kintner et al., 2007). When the astrocytes are returned to physiological control buffer, [Na+]i rapidly increased at a rate of 42 mM min−1 reaching a peak value of ~75 mM. The [Na+]i then declined at ~5 min and plateaued during the 30 min recovery (Kintner et al., 2007). Bumetanide or genetic ablation of NKCC1 reduces this hypoxia-mediated rise in [Na+]i by ~65% (Kintner et al., 2007). The HAIR-mediated increases in [Na+]i is doubled when Na+/K+-ATPase activity is blocked with ouabain, suggesting that under hypoxic conditions NKCC1-mediated Na+ overload cannot be compensated by Na+/K+-ATPase activity (Kintner et al., 2007). These findings suggest that deregulated NKCC1 following ischemia causes intracellular Na+ overload which can affect cell demise by directly causing damage, oxidative stress, and apoptotic cell death (Banasiak et al., 2004).
NKCC1-mediated increase in [Na+]i following in vitro ischemia has been linked to disruption of Ca2+ homeostasis in astrocytes (Lenart et al., 2004). The mechanism of ischemic injury in astrocytes causes an excessive Ca2+ influx and Ca2+ release from intracellular stores, triggering Ca2+-mediated cell death cascades (Bano and Nicotera, 2007). As mentioned earlier, increase in [Na+]i leads to the activation of reverse mode function of NCX which causes increases in [Ca2+]i (Smith et al., 2000) and sequestration of intracellular Ca2+ by the ER and mitochondria. As a result, a twofold rise in intracellular Ca2+ release from the bradykinin-sensitive ER stores was detected in astrocytes (Lenart et al., 2004). Inhibition of NKCC1 activity as well as inhibition of reverse mode operation of NCX with KB-R794 significantly lowered the Ca2+ release (Lenart et al., 2004). These findings suggest that reverse mode operation of NCX activated by increased Na+ influx by NKCC1 and/or NHE1 activity contributes to Ca2+-mediated astrocyte damage. NKCC1 activity in astrocytes also contributes to excitotoxic injury by increasing swelling-induced glutamate release and decreasing glutamate uptake from the extracellular space (Abdullaev et al., 2006; Su et al., 2002a). Pharmacological inhibition of NKCC1 or deletion of NKCC1 in astrocytes decreased the release of aspartate (Su et al., 2002a). Taken together, these data show that NKCC1-mediated breakdown of Na+ gradients plays a significant role in the early stages of ischemic cytotoxic damage.
Besides perturbation of Na+ homeostasis, NKCC1-mediated regulation of intracellular Cl− homeostasis is also imbalanced in ischemic astrocytes. A significant increase in intracellular Cl− content was detected in astrocytes subjected to OGD/Reoxygenation which was abolished by inhibition of NKCC1 with bumetanide (Lenart et al., 2004). Thus, the cumulative effects of uncontrolled Na+ and Cl− influx can result in increase in intracellular osmolarity, driving water influx and astrocyte swelling (Fig. 1).
Among the mechanisms involved in the ischemia-induced increase in NKCC1 activity, phosphorylation dependent stimulation of NKCC1 has been identified in astrocytes. Lenart et al., (2004) measured NKCC1 activity in astrocytes by the extent of its phosphorylation and also functionally by measuring 86Rb influx rate after OGD/Reoxygenation. Sustained increase in NKCC1 activity was detected in astrocytes after OGD/Reoxygenation, which was associated with increased expression of phosphorylated NKCC1 protein (~300% increase) (Lenart et al., 2004; Su et al., 2002a,b). Besides phosphorylation, elevated extracellular potassium ([K+]o) (> 60 mM) levels, which occur in cerebral ischemia, are also known to stimulate NKCC1 activity in neurons and astrocytes (Su et al., 2002a,b). The high [K+]o induced activation of NKCC1 was abolished by removing extracellular Ca2+ or blocking the voltage gated Ca2+ channels, suggesting that NKCC1 activity is stimulated via Ca2+ mediated signal transduction pathways under high [K+]o. WNK-SPAK-OSR1 serine-threonine kinases are known to be responsible for stimulation of NKCC1 phosphorylation and activation in neurons and oligodendrocytes (Begum et al., 2015), but their role in astrocytic NKCC1 regulation remains to be established.
Astrocyte swelling, brain edema, and the subsequent increase in intracranial pressure and brain herniation are complications of ischemia (Kahle et al., 2009; Liang et al., 2007). Astrocytes are more prone to swelling than neurons as they are involved in K+ clearance and glutamate uptake, which cause osmotic overload that in turn promotes water influx. Su et al. (2002a,b) demonstrated that high [K+]o-induced activation of NKCC1 triggers 20% cell swelling in astrocytes, which was abolished by inhibiting NKCC1 activity with bumetanide or by genetic ablation (Su et al., 2002a,b). Inhibition of NKCC1 activity in BBB endothelial cells also causes reduced cell damage (O’Donnell et al., 2005). In corroboration with above findings, increased NKCC1 protein and mRNA expression are found in cortex, striatum and hippocampus following middle cerebral artery occlusion and 24 h reperfusion (Yan et al., 2001; Kang et al., 2002). Intracerebral bumetanide administration via microdialysis probe during ischemic stroke inhibits NKCC1 activity and potently reduced neuronal and astrocyte swelling, thereby reducing the overall infarct volume and brain edema (Yan et al., 2001, 2003; Staub et al., 1994). Furthermore, genetic ablation of NKCC1 in mice displays reduction in brain edema upon transient ischemic stroke (Chen et al., 2005).
Activation of NKCC1 is a mechanism of astrocyte swelling/brain edema also in other neurological disease conditions (Jayakumar et al., 2008, 2011; Lu et al., 2006; Norenberg, 1987). Collectively, these findings suggest that perturbations of Na+, Cl−, K+, and Ca2+ ion homeostasis during hypoxia/ischemia by uncontrolled NKCC1 activity can severely damage the astrocytes and lead to neurological dysfunction.
Oligodendrocytes
Oligodendrocyte in periventricular white matter and white matter tracts in spinal cord abundantly express NKCC1 (Jantzie et al., 2015; Plotkin et al., 1997b). The resting [Na+]i in oligodendrocytes is found to be ~18.4 mM. Application of AMPA results in increased phosphoactivation of NKCC1 which further enhances intracellular Na+ overload (Chen et al., 2007). Stimulation of AMPA receptors results in ~7.2 fold increase in [Na+]i, from 18.8 to 134 mM, and inhibition of NKCC1 activity by bumetanide reduced the [Na+]i rise by ~53% (Chen et al., 2007). The data suggest that the significant portion of Na+ entry was associated with NKCC1 activation (Chen et al., 2007). AMPA receptor-mediated activation of NKCC1 also led to sustained increase in [Ca2+]i and resulted in mitochondrial Ca2+ accumulation, followed by release of cytochrome C and cell death (Chen et al., 2007). The sustained increase in cytoplasmic Ca2+ was abolished by NCXrev inhibitor KB-R7943 and by inhibition of NKCC1 with bumetanide. These findings demonstrated that NKCC1 and NCXrev work together in the AMPA- mediated Ca2+ signaling in oligodendrocytes. Inhibition of NKCC1 activity or NCXrev, blocked the AMPA-mediated excitotoxic damage in oligodendrocytes. Studies have shown that AMPA/Kainate receptor-mediated excitotoxicity results in spinal cord white matter injury in a process that is linked to oligodendrocyte damage and axonal demyelination wherein activation of NKCC1 could not be ruled out (Park et al., 2004; Tekkok and Goldberg, 2001). Increase in intracellular Na+ and subsequent NCXrev mediated activation of Ca2+ signaling pathways is also shown to cause structural and functional axonal injury (Stys, 2004). Taken together, these studies suggest that NKCC1 plays an important role in white matter damage. Ischemic injury of developing white matter is a common pathology associated with disorders of cerebral palsy (Wilke et al., 2004). NKCC1 is known to play a role in ischemia-induced acute death of immature oligodendrocytes. Increase in cytoplasmic Ca2+ was detected in rat optic nerve oligodendrocytes in situ exposed to OGD (Fern et al., 1995). Ischemia-mediated increased activity of astrocytic NKCC1 leads to excessive release of glutamate via Na+-mediated glutamate transporters. Released glutamate subsequently activates non-NMDA glutamate receptors on oligodendrocytes, gating a toxic influx of Ca2+ and causing acute cell death (Wilke et al., 2004). Indeed, removing extracellular Ca2+ or blocking NKCC1 activity with bumetanide limited the Ca2+ influx and protected oligodendrocytes from injury, suggesting that inhibiting NKCC1 activity provides protection to the developing white matter after ischemic insults (Wilke et al., 2004).
A very recent study has revealed that OGD/Reoxygenation induced increase in phosphoactivation of NKCC1 by WNK3-SPAK/OSR1 kinases in OPC. OPC death was prevented by pharmacological inhibition of NKCC1 with bumetanide or gene deletion of NKCC1 kinase WNK3. Deletion of WNK3 or siRNA knockout of SPAK/OSR1 kinases also increased OPC’s tolerance to OGD and conferred significant protection (Begum et al., 2015). In addition, increased expression of pNKCC1 and pSPAK/pOSR1 was seen in APC-positive mature oligodendrocytes in injured white matter at 72 h following ischemic stroke. Gene deletion of WNK3 or SPAK/OSR1 kinases significantly reduced demyelination of corpus callosum and promoted recovery of sensory motor functions (Begum et al., 2015; Zhao et al., 2016). Collectively, these experimental data strongly suggest a role for NKCC1 in white matter damage following ischemia (Fig. 1).
Oligodendrocytes are the principal cells affected by pathophysiology of periventricular leukomalacia (PVR). PVR is a gestation-dependent white matter lesion found in preterm infants and a major precursor of cognitive impairment and cerebral palsy (Volpe, 2003). A rodent model of PVR shows that NKCC1 is highly expressed in oligodendrocytes during early development and increases white matter’s vulnerability to hypoxic ischemic injury (Jantzie et al., 2015). Inhibition of NKCC1 activity with bumetanide conferred significant protection against white matter injury (Jantzie et al., 2015). Taken together, these results suggest that like neurons oligodendrocytes are highly sensitive to ischemic damage and understanding the mechanisms of oligodendrocyte death may suggest new therapeutic strategies to preserve or restore white matter function and structure after ischemic insults.
Na+/K+-ATPase
The Na+/K+-ATPase is responsible for maintenance of the resting membrane potential (Watts et al., 1991). Although the ATP shortage experienced during cerebral ischemia would suggest that inhibition of this transporter determines a worsening of the ischemic lesion, the experimental data are conflicting, and to date, the exact role of this protein has not yet determined in stroke (Kaplan, 2002; Scheiner-Bobis, 2002); probably a key point in this debate is the role played by this antiporter in controlling Na+ dynamics in glial cells.
Astrocytes
The composition of this protein is regulated in both a developmental and cell-specific manner (Watts et al., 1991). The α2-subunit of the Na+/K+-ATPase has been almost exclusively found to be expressed in astrocytes where it colocalizes with both glial glutamate aspartate transporter and glutamate transporter-1 on fine astrocytic processes surrounding glutamatergic synapses (Cholet et al., 2002). Notably, the Na+/K+-ATPase, bearing α2 subunits, has been found to colocalize with NCX in cortical astrocytes at plasma membrane–ER junction level, where tightly regulated ’sodium microdomains’ may be present (Blaustein et al., 2002). Although it has been shown that after hypoxia an increase in the expression of Na+/K+-ATPase α1 and β1 subunits occurs in astrocytes during the reoxygenation phase, they may not be able to maintain the typical plasmamembrane function of the pump for the lack of ATP (Leis et al., 2005). In fact, immediately after ischemia induction, despite the occurrence of an increase in Na+/K+-ATPase expression (Leis et al., 2005), its activity is suppressed (Peng et al., 1997; Pimental et al., 2013). In effect, there is a substantial heterogeneity among reactive astrocytes, with some close to the ischemic lesion showing decreased buffering capacity and those in the penumbra region showing a higher ionic buffering ability (Leis et al., 2005).
Microglia
The Na+/K+-ATPase is not functionally active in microglia, however, H+/K+-ATPase seems to vicariate its role in these glial cells (Shirihai et al., 1998).
Oligodendrocytes
Oligodendrocytes express the alpha 1 and alpha 2 isoforms of the Na+/K+-ATPase catalytic subunit (Fink et al., 1996) and represent the unique glial cells that express the β3 isoform (Martin-Vasallo et al., 2000).
No studies have investigated the role of oligodendroglial Na+/K+-ATPase in in vivo or in vitro models of stroke, however, since this pump is strongly activated by increasing [K+]o (Dobretsov and Stimers, 1996), it is possible to hypothesize that this transporter is activated in this cell type after brain ischemia.
NBC
Na+-HCO–3 cotransporter, represents, together with NHE, one of the main cellular system involved in the regulation of ionic and pH homeostasis in the CNS where it can transport either two or three bicarbonate ions per one sodium ion (Soleimani and Burnham, 2001) An increased expression of NBC1 in the ischemic penumbra has been detected in a rat model of focal cerebral ischemia (Jung et al., 2007). Although the reason for this ischemia-induced increase in NBC1 expression is not known, the authors suggest that ischemia-mediated increase in K+ depolarizes astrocytes and stimulates NBC1 activity. The increased cellular influx of HCO–3 could buffer ischemia-mediated intracellular acidosis. In contrast, the accompanying Na+ influx is expected to promote cellular edema (Ostby et al., 2009). Consistent with this observation, Cooper et al., (2009) reported increased expression of NBC1 in cultured hippocampal neurons under ischemic conditions induced by low pHo and absence of extracellular Mg2+. In particular, glutamate cytotoxicity was decreased in neurons from NBC1 siRNA knockdown animals after incubation in Mg2+ free media but not when cells were incubated at low pHo of 6.3. The authors speculated that 0 Mg2+ stimulates NMDA receptor activity, which in turn induces cytotoxicity promoted by NBC1-mediated acid extrusion (Cooper et al., 2009).
Astrocytes
Among the three NBC1 splice variants (A, B, and C), NBC1-B and likely -C are the predominant variants expressed in rat brain astrocytes (Majumdar and Bevensee, 2010). Several evidences reported that NBC in astrocytes may modulate neuronal excitability through pH changes. In fact, when a neuron fires an action potential, there is an increase in extracellular K+ that depolarizes neighbouring astrocytes. This depolarization leads to stimulation of NBC activity in astrocytes that in turn induces an increase in Na+, HCO–3 and net-negative charge into the cells. The ensuing decrease in pHo tends to dampen further neuronal activity by inhibiting pH-sensitive, voltage- and ligand-gated channels. This negative-feedback model is predicted to be neuroprotective under pathophysiological conditions associated with ischemia. Some studies carried out in in vivo models of stroke suggest a not well explained detrimental role of glial NBC overexpression (Jung et al., 2007). Indeed, the absence of immunoreactivity for astrocytic NBC in the CA3 hippocampal region is associated to the recognized resistance of this brain region to the ischemic insult (Sohn et al., 2011).
Microglia
Na+/HCO3− cotransporter is expressed in microglia (Faff et al., 1996), but its role in brain ischemia seems to be less relevant than in astrocytes (Sohn et al., 2011).
Oligodendrocytes
The Na+-dependent HCO–3 influx mechanism is known to be involved in pHi homeostasis of cultured oligodendrocytes (Boussouf et al., 1997; Kettenmann and Schlue, 1988). In the presence of external bicarbonate/CO2, resting pHi is not modified by the removal of external chloride or blockers of the chloride-coupled Cl−/HCO3 transport system. The pHi regulation in mature oligodendrocytes is exclusively dependent on the Na+ gradient (Boussouf et al., 1997; Lascola and Kraig, 1997) speculated that changes in the expression of NBCs could be associated with dysregulation of pHi and/or intracellular Na+ concentration. Spatial nonuniformity of pH in oligodendrocytes is generated by differential subcellular distribution of NHE, NBC, and carbonic anhydrase II, which colocalize with either NHE or NBC. Therefore, the changes in the expression of NBCs could be associated with dysregulation of pHi and/or intracellular Na+ concentration and this may play a role in the pathophysiological events seen in brain ischemia (Lascola and Kraig, 1997).
The proper functioning of pH regulatory NBC are critical for neuronal function, although the transporters appear to be present primarily in glial cells. The unique roles of different NBCs in specific cell contexts under ischemia and other neurological disorders remains to be further investigated.
Alzheimer’s Disease and Other Dementias
Growing evidence indicates that the early disruption in neuron-glia signaling significantly contributes to synaptic and cognitive impairment in Alzheimer’s disease (AD) (Chung et al., 2015). The emerging role of astrocytes and microglia in synaptic development and synapse homeostasis in the healthy brain further suggests the pivotal role of these cells in the early dysfunction of synapses in AD (Paolicelli et al., 2011). Indeed, atrophy of astrocytes is believed to give a direct contribution not only to synaptic pathology, but also to the inflammatory responses, and the evolution of amyloid plaques (Nedergaard and Verkhratsky, 2012; Verkhratsky et al., 2010). Although a large number of studies recognize the importance of microglia in mediating the inflammatory response in the AD brain (Rodríguez et al., 2016), in the last few years, a number of genes involved in microglial biology have been identified in AD genetic association studies, thus suggesting their potential role in disease etiology (Lambert et al., 2013; Paolicelli et al., 2011). Very recently, Hong et al. (2016) demonstrated that microglia and immune-related pathways can act as early mediators of synapse loss and dysfunction that occur in AD models before plaques form.
The role of oligodendrocytes lineage cells in AD pathology remain less well defined, although there is an extensive literature documenting the contribution of white matter damage to cognitive decline in AD brains (Desai et al., 2010). Emerging studies suggest that glial Na+-dependent transporters may be implicated in synaptic dysfunction and excitotoxic damage in AD.
Na+/K+-ATPase
The observation that mice lacking the α2 isoform of Na+/K+-ATPase, which is the isoform mainly represented in astrocytes, die soon after birth (Ikeda et al., 2004), highlights the importance of the glial Na+/K+-ATPase. The pivotal role played by the glial Na+/K+-ATPase expressing the α2 subunit in cognitive functions was first suggested by Moseley et al. (2007) in a study showing that heterozigous mice deficient in Na+/K+-ATPase α2 gene displayed spatial learning and memory deficits. Consistently, a significant perturbation of Na+ and K+ ions has been described in post-mortem AD brain tissue (Vitvisky et al., 2012) and a significant reduction of the Na+/K+-ATPase protein levels and activity was previously observed in the brain of both AD patients (Liguri et al., 1990; Chauhan et al., 1997; Hattori et al., 1998) and APP+ PS1 transgenic mice (Dickey et al., 2005).
Astrocytes
The fundamental contribution of the astrocytic Na+/K+-ATPase to the ionic imbalance observed in AD is evidenced by the findings showing that, in cultured astrocytes, both acute and repeated Aβ25–35 exposure cause a significant increase in intracellular Na+ and K+ levels that is associated with reduced protein levels and activity of Na+/K+-ATPase (Vitvisky et al., 2012). The alteration of Na+/K+-ATPase activities in neurons of AD brains may, at least in part, result from the cytotoxic effects of Aβ proteins, and has been mostly related to the pathophysiology of neuronal excitotoxicity (Ohnishi et al., 2015). By contrast, emerging data suggest that the impaired functionality of Na+/K+-ATPase in astrocytes maybe be involved into the mechanisms leading the brain glucose hypometabolism observed in pre-symptomatic AD (Mistur et al., 2009). In fact, the highly localized astrocytic Na+/K+-ATPase containing the α2 subunit participates in a regulated metabolic cooperation process between neurons and astrocytes, as it is functionally coupled to glucose uptake and lactate production following glutamate uptake into astrocytes (Pellerin and Magistretti, 1994; Rose and Chatton, 2016; Takahashi et al., 1995). The regional hypometabolism of glucose in the brain is considered as an hallmark of AD. For instance, fluorodeoxyglucose positron emission tomography studies have shown a consistent and progressive reduction of glucose utilization in AD patients that can even precede the onset of symptoms (Mosconi et al., 2008). In addition, evidence have been provided indicating that unaffected individuals at risk for developing AD displayed a decrease in glucose metabolism in astrocytes from selected brain areas (Magistretti and Pellerin, 1996; Nilsen et al., 2014). Based on these observations it can be hypothesyzed that the impaired functionality of Na+/K+-ATPase activities may contribute to the altered glucose metabolism observed in these individuals, and possibly to the astrocyte dysfunction observed at early stage of AD disease.
NHE
NHE1-mediated alteration of pHi homeostasis has been reported in patients exhibiting AD pathology (Urcelay et al., 2001). The neurotoxic effects of β amyloid peptide in AD have been related to its capacity to perturb the cellular Ca2+ homeostasis (Ibarreta et al., 1997). The generation of amyloid fibrils in vitro has shown to be pH dependent (Otvos et al., 1993). Moreover, intracellular acidification accompanied with elevated intracellular free Ca2+ contributes to neuronal death in AD (Ibarreta et al., 1998; Khachaturian, 1993; Siesjo, 1981). In contrast, increase in proliferation of lymphoblasts and intracellular alkalization was also detected in AD patients and was found to be abolished by NHE inhibitor EIPA (Urcelay et al., 2001). These findings suggest that NHE-mediated disruption of pHi homeostasis is involved in the pathogenesis of AD.
Astrocytes
Benos et al. (1994) have reported the role of astrocytic NHE1 in the development of AIDS-mediated dementia. Exposure of primary astrocyte cultures to HIV-linked gp-120 and cytokines induced astrocyte proliferation and NHE1 activation. This study suggests that activated NHE1 in astrocytes increases the pHi and subsequently enhances the release of glutamate via reversal of Na+-dependent glutamate transporters. Increased extracellular glutamate causes Na+ and Ca2+-mediated excitotoxic damage to surrounding neurons and neuronal damage and dementia (Benos et al., 1994). This hypothesis of NHE in dementia was further supported by a later study that the gp-120 triggers the activation of NHE1 and Ca2+-mediated neurodegeneration (Holden et al., 1999).
Although emerging studies suggest a pivotal role of the neuronal NCX3 exchanger in regulating Ca2+ homeostasis in AD (Pannaccione et al., 2012; Sokolow et al., 2011), no evidence has been provided to address the role of other Na+-dependent transporters in AD.
Epilepsy
Epilepsy is a chronic disorder of brain function characterized by periodic and unpredictable onset seizures. Recent evidence indicates a critical role of glia dysfunction in the pathogenesis of this neurological disease (Seifert et al., 2006; Coulter and Steinhauser, 2015).
Astrocytes
Astrocytes take part to the early stage of the pathology, when they increase in number and change their shape becoming hypertrophic. This astrogliosis has been described temporarily before the appearance of fully developed seizures (Carmignoto and Haydon, 2012). These morphological changes have been attributed to changes in K+ homeostasis and in aquaporin and glutamate transporter expressions, all events that are tightly linked also to astrocytic Na+ dynamics. Notably, the Na+ dynamic in astrocytes can be easily linked to that of glutamate that is massively released in the synaptic wall during epilepsy. In fact, astrocyte glutamate transporters provide most of the glutamate clearance in the brain and take up glutamate along with Na+ ions (Danbolt, 2001; Rose and Ransom, 1996). Therefore, synaptic release of glutamate is closely coupled to a cytosolic Na+ increase in astrocytes (Kirischuk et al., 2012; Langer and Rose, 2009).
Importantly, it has been shown that a short increase of neuronal activity of 200 ms is sufficient to induce in hippocampal astrocytes in situ vigorous Na+ transients, which can last about 10 s (Langer and Rose, 2009). This increase of Na+ does not take place in a homogeneous way: the soma of astrocytes presents variations of the concentrations of Na+ less marked than the peripheral processes that are more closely in touch with the synaptic structures. A peculiarity of these local increases of Na+ is the propagation capacity to neighbor astrocytes (Karus et al., 2015; Langer and Rose, 2009). In contrast, an increase of cytosolic Na+ levels can have substantial effects on astrocyte physiology (Kirischuk et al., 2012). In fact, 12 mM increase of intracellular Na+ reduces the driving force for glutamate uptake by about 20% (Karus et al., 2015). At the same time, a Na+ increase of this magnitude could easily reverse GABA uptake in astrocytes and release this inhibitory neurotransmitter (Kirischuk et al., 2012; Richerson and Wu, 2003). Similarly, the extracellular concentration of the NMDA receptor co-agonist glycine and of its transporter Glyt1b, is influenced by high level of intra-astrocytic Na+ (Roux and Supplisson, 2000). This increase in astrocyte Na+ can have a dual effect, since it may increase NMDA receptor mediated excitation, but at higher concentrations it can also induce an inhibitory effect mediated by glycine receptor activation. Glyt1 blockade was recently shown to inhibit epileptic activity (Shen et al., 2015) suggesting that elevated extracellular glycine levels may overall inhibit seizures. Finally, Na+ increase in astrocytes can trigger the reversal of the Na+/Ca2+ exchanger, effectively allowing Ca2+ to enter the astrocyte (Kirischuk et al., 1997), thereby linking Na+ entry to the Ca2+-dependent mechanisms. From the above mentioned studies, the increase of Na+ in astrocytes may determine either a protective or detrimental role against seizure. Two recent reports would favor the detrimental role, indeed an experimentally induced intra-astrocyte [Na+] increase by approximately doubling the resting [Na+]i over an hour induces epileptiform discharges within 30 min in vivo (Willoughby et al., 2003) and in vitro (Karus et al., 2015). Thus, astrocyte Na+ signaling in response to neuronal epileptic activity may be involved in establishing a period of increased excitability by positive feedback or synchronization.
Little information is available on the nature of the proteins involved in the maintenance of Na+ homeostasis during epilepsy in other glial cells.
NCX
Although selective studies on the role of glial NCX isoform subtypes in epilepsy have not been performed, results obtained in brain rodents showed that NCX activation may inhibit pharmacologically induced convulsions (Saito et al., 2009).
Interestingly, a recent study reports that point mutations in the Drosophila cortex of glial K+-dependent Na+/Ca2+ Exchanger, NCKX, another member of NCX superfamily, eliminates Ca2+ oscillations in microdomains of cortical glia and predisposes to seizures in response to several environmental stressors (Melom and Littleton, 2013). Thus, since the impairment of NCKX in astrocytes triggers rapid neuronal seizures, glial NCKX represents the first identified glial-specific gene responsible for an epileptic phenotype (Melom and Littleton, 2013).
NKCC
A prominent role in epileptic development seems to be played by the glial NKCC1. NKCC1 expression is developmentally regulated in the human and rodent brain with increased expression in neurons and glial cells during early developmental stages and declining in late adulthood (Dzhala et al., 2005; Kaila et al., 2014). The increased levels of NKCC1 expression in neurons and glia are known to reduce GABAergic signaling causing increased excitability. Therefore, NKCC1 is being explored as a target for anticonvulsant therapy in neonates (Cleary et al., 2013; Dzhala et al., 2005; Kaila et al., 2014) and for other developmental diseases (Nepomuceno and Sun, 2015; Wu and Sun, 2015).
Microglial activation has been shown to have indirect effects on NKCC1 activity in neurons, thereby affecting their excitability (Wake et al., 2007). During epileptic seizures, microglia become activated and may secrete BDNF. By binding to TrkB receptors on neurons, secreted BDNF triggers intracellular signaling cascades which increase Cl− influx through NKCC1 overexpression but decreases Cl− efflux through KCC2 overexpression in neurons (Wake et al., 2007). These changes consequently disrupt the Cl− homeostasis and reduce GABA-mediated inhibitory function, which leads to epilepsy or neuropathic pain (Coull et al., 2005; Rivera et al., 2002). Blockade of microglial BDNF–TrkB signaling has been shown to restore normal Cl− homeostasis in neurons by normalizing the expression of NKCC1 and KCC2 (Cordero et al., 2005), thus preventing epilepsy.
Parkinson’s Disease
Activation of astrocytes and microglia are common features observed both in Parkinson’s disease (PD) patients and in animal models of PD. Both glial cell types contribute to the neuroinflammatory processes associated with PD (Verkhratsky et al., 2014; Wang et al., 2015).
The role of astrocytes in PD is not well understood (Verkhratsky et al., 2014). Similar to microglia, the role of astrocytes has been mostly related to the release of neuroprotective/harmful mediators. Activated microglia appears to play a beneficial role only during the early stage of the disease. By contrast, its excessive activation during late stages leads to the release of proinflammatory mediators that accelerate the degeneration of dopaminergic neurons (Norden et al., 2015).
Although α-synuclein-containing inclusions have been also observed in oligodendrocytes, very little information is available on the their role during PD (Campbell et al., 2001).
Very few studies investigated the involvement of glial Na+-dependent transporters in the pathophysiology of PD disease.
Na+/K+-ATPase
Both dysregulation and reduced activity of the Na+/K+-ATPase has been observed in PD and rapid-onset dystonia Parkinsonism (RDP) (Cannon, 2004; Chauhan et al., 1997; de Carvalho et al., 2004). The importance of this pump in PD pathophysiology is revealed by the observation that mutations in the Na+/K+-ATPase alpha3 gene atp1a3 are associated with rapid-onset dystonia parkinsonism (de Carvalho et al., 2004; Rodacker et al., 2006).
NKCC
Recently, a clinical study reports that the NKCC1 blocker bumetanide was able to provide protection in patients affected by PD (Damier et al., 2016). Considering the critical role of NKCC1 in controlling Na+ homeostasis in neurons and glial cells, it would be important to explore in future studies the functional role of this cotransporter in PD pathogenesis.
Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is one of the most debilitating neurodegenerative diseases. It involves motor neurons degeneration resulting in the impossibility to carry out normal functions. The cause of this disease has not yet been completely understood, however, it is known that only 5% of all cases of ALS are attributable to genetic mutations. Among them, 20% is due to a mutation in the superoxide dismutase 1 (sod1) gene.
Notably, glial cells such as astrocytes, microglia and oligodendrocytes contribute to non–cell autonomous motor neurons (MN) death in ALS (Ilieva et al., 2009; Philips and Rothstein, 2014). The role of [Na+]i and of the proteins that influence Na+ homeostasis in glia-mediated MN death has been extensively hypothesized in several excellent reviews, however, the only data available are related to astrocytic Na+ homeostasis in ALS, while no data are available for microglia and oligodendrocytes.
Na+/K+-ATPase
It has been established that an overexpression of the Na+/K+ ATPase in spinal cord astrocytes of G93A sod1 mice is deleterious and aggravates the burden of the disease (Gallardo et al., 2014). This hypothesis has been confirmed by experiments showing that the pharmacological blockade, the silencing or the genetic ablation of the Na+/K+-ATPase in the astrocytes of animals bearing the G93A sod1 mutation, ameliorates ALS consequences and prolongs lifespan. In addition, an overexpression of this transporter has been detected in the spinal cord of patients affected by ALS (Gallardo et al., 2014). The reason for the putative detrimental role of Na+/K+-ATPase has not clearly established. However, it is reported that Na+/K+-ATPase overexpression triggers mitochondrial respiratory chain in mutant sod1 astrocytes. This effect seems to be due to the increase in mitochondrial Na+ concentrations that is restored by the activation of mitochondrial NHE, hence reducing the proton gradient necessary for the respiratory chain (Bernardinelli et al., 2006). Mitochondrial respiration may increase ROS in mutant sod1 astrocytes, which in turn may activate the induction of inflammatory factors leading to non–cell autonomous degeneration of motor neurons (Di Giorgio et al., 2008; Gallardo et al; 2014). Furthermore, it is possible to speculate that the overexpression of Na+/K+-ATPAse described in astrocytes of ALS animals triggers a reduction of [Na+]i that can have the following consequences: (1) the entrance of Na+ through NCX operating in the forward mode, with consequent increase of [Ca2+] in the synaptic wall; (2) an increase in the synaptic glutamate concentrations due to astroglial swelling that decreases extracellular volume, hence increasing concentration of neurotransmitters and damage signals in the interstitium, and to a reduction of glutamate uptake by EAAT, whose expression is dependent on [Na+]i and (3) the reduction of astrocyte-neuron lactate shuttle, as astrocytes provide the metabolic substrate lactate to neurons in a Na+-dependent manner (Ferraiuolo et al., 2011; Marchetto et al., 2008).
In contrast, the reason for the Na+/K+-ATPase overexpression could be the necessity of the cells to extrude the excess of intracellular Na+ due to the excessive release of glutamate that in turn activates the Na+/glutamate transporter on astrocytes. In fact, glutamate activates ionotropic receptors and glutamate/Na+ transporters, which results in a net Na+ influx; the latter can increase intracellular Na+ concentration from the resting level of ~5 mM to 20–30 mM. This increase is counteracted by rapid reversal of the NCX, which expels the excess of Na+ and provides Ca2+ entry, and a slower Na+ extrusion through the Na+/K+ pump, which is energy-dependent (Verkhratsky, 2013).
Further studies are needed to establish Na+ dynamics in the different astrocytic phenotypes during ALS.
Myelin Disease of the CNS
Recently, the functional involvement of specific oligodendroglial and astroglial Na+ transporters has been described in distinct myelin disease of the CNS encompassing (1) demyelinating, and (2) dismyelinating or hypomyelinating diseases.
Demyelinating Diseases: Multiple Sclerosis
The loss of myelin in demyelinating diseases is the consequence of a primary attack to oligodendrocytes, the myelin-forming cells, caused by an inflammatory disease, viral infection or toxic insult. Multiple sclerosis (MS) is the most common chronic inflammatory demyelinating disease of the CNS, characterized by an aberrant immune response leading to axonal demyelination and neurodegeneration (Compston and Coles, 2008). Despite the ability of the OPC to generate oligodendrocytes with myelination capacity, remyelination of undamaged axons in MS lesions fails or is incomplete and no neuroprotective or remyelinating therapies are still available (Chong and Chan, 2010).
Polarized phenotypes of microglia play distinct roles during MS disease. M1 microglia is responsible for antigen presentation, phagocytosis of myelin and release of proinflammatory molecules in the early stages of the disease. Protective M2 microglia dominates at later stages of the demyelination process and sustains the recruitment of OPC in repair mechanism and remyelination (Miron et al., 2013).
Astrocytes also are capable of antigen presentation and release inflammatory molecules which have profound effects both on resident and infiltrating immune cells. Although astrocytes have long been considered to inhibit CNS repair via formation of the glial scar, it is now recognized that astrocytes regulate myelin formation (Nash et al., 2011).
NCX
Oligodendrocytes
Recently, the Na+/Ca+2 exchanger NCX3 is emerging as an important player in the regulation of [Na+]i and [Ca2+]i in oligodendrocytes during physiological and demyelinating conditions, i.e. during myelin oligodendrocyte glycoprotein (MOG)33–55- induced EAE, an animal model of MS (Annunziato et al., 2013; Boscia et al., 2012; Casamassa et al., 2016). Our findings, along with that of other investigators, indicates that NCX3 is a protein component of the myelin membrane (Gopalakrishnan et al., 2013) and that calcium and sodium signaling mediated by this exchanger plays an important role in oligodendrocytes differentiation (Boscia et al., 2012, 2013). Indeed, whereas the knocking down of the NCX3 isoform in OPC prevents the up-regulation of the myelin protein markers 2′,3′-cyclic-nucleotide 3′-phosphodiesterase (CNPase) and myelin basic protein (MBP), its overexpression induces an up-regulation of both CNPase and MBP (Boscia et al, 2012, 2013). More recently, we demonstrated that NCX3 is significantly upregulated in oligodendrocyte lineage cells of the white matter spinal cord during EAE disease and it is crucially involved in oligodendrocyte response after MOG35–55-induced demyelination (Casamassa et al., 2016). Consistently, we found that mice lacking ncx3 gene displayed increased susceptibility and developed more severe clinical symptoms after MOG35–55-induced EAE. The exacerbated development of EAE disease in ncx3−/− mice was accompanied by a significant reduction in OPCs and premyelinating cells in the spinal cord during the chronic stage if compared with congenic ncx3+/+ mice (Fig. 3, modified by Casamassa et al., 2016). By contrast, no significant difference was observed between the number of immune cell subsets present in ncx3+/+ and ncx3−/− mice during the peak stage of the disease, thus implying that it is the absence of NCX3 in the cell population of the CNS and not in the lymphoid cells that may account for the EAE phenotype observed in ncx3−/− mice (Casamassa et al., 2016). These results are in line with the evidence that ncx3−/− mice have reduced spinal cord size and decreased expression of myelin markers (Boscia et al., 2012).
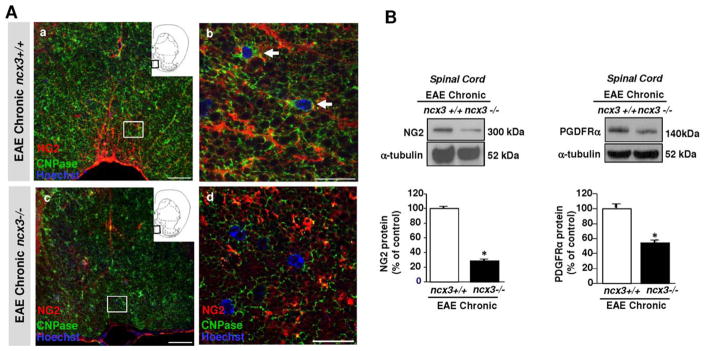
FIGURE 3.
Impaired oligodendrocyte lineage response in the spinal cord of EAE mice lacking ncx3 gene. A, Confocal double immunofluorescence images displaying at a lower (a, c) and higher (b, d) magnification NG2 (red) and CNPase (green) distribution in the white matter of ncx3+/+ (a–b) and ncx3−/− (c–d) mice in the chronic stage of EAE disease. NG2/CNPase double-labeled cells are frequently observed in the white matter of ncx3+/+ mice (arrows in b), but not in ncx3−/− mice. Scale bars: 50 μm in a and c; 20 μm in b and d. B, Western blotting and densitometric analysis of NG2 (left panel) and PDGFα receptor (right panel) protein levels in spinal cord lysates from control and MOG35–55-immunized mice in the chronic stage of EAE. Data were normalized on the basis of α-tubulin and expressed as percentage of controls. The protein levels of both NG2 and PDGFα receptor, two proteins that are characteristically co-expressed by OPCs, are significantly downregulated in the spinal cord homogenates from ncx3−/− mice when compared with ncx3+/+ mice. The values represent the means±S.E.M. (n=3–4). *P < 0.05 versus controls. This figure was modified by Casamassa et al., 2016, Glia, 64:1124–1137.
Na+/K+-ATPase
Although no studies have been carried out to determine the role of Na+/K+-ATPase in the distinct glial cells, Giatti et al. (2013) reported that modifications of the the Na+/K+-ATPase enzymatic activity occur during EAE induced in rats with syngenic whole spinal cord homogenate. In particular, a significant decrease in the Na+/K+-ATPase function was observed during both the acute and the chronic phases of EAE disease.
Dismyelinating or Hypomyelinating Diseases: Leukodystrophies
Dysmyelination or hypomyelination processes are associated with distinct leukodystrophies, a group of congenital myelin disorders characterized by defects in genes encoding myelin proteins, or enzymes required for myelin biogenesis and maintenance, or by defects in genes expressed in astrocytes or associated with specific astrocyte function (Lanciotti et al., 2013). For instance, the myelin degeneration observed in Alexander’s disease, megalencephalic leukoencephalopathy with subcortical cysts (MLC) and vanish white matter (VWM) occur as a consequence of a primary astrocyte defect (Lanciotti et al., 2013).
Na+/K+-ATPase
Astrocytes
Dysregulation of the astroglial MLC1-Na+/K+-ATPase complex may contribute to the pathological events leading to myelin disturbance occurring in MLC disease. MLC is a rare autosomal recessive leukodystrophy characterized by macrocephaly, brain edema, subcortical cysts, myelin and astrocyte vacuolation (De Keyser et al., 2008). Mutations in either the mlc1 gene (75%) or the GlialCAM gene (20%) account for the disease. Recently, the GlialCAM adhesion protein was found essential for the expression and function of the chloride channel ClC-2; by contrast, the identity and the exact functional role of MLC1 protein, which is not expressed in oligodendrocyte but highly represented in astrocyte processes is yet not known (Boor et al., 2005). By using complementary biochemical, proteomic, and protein interaction assays in cultured astrocytes and brain tissue Brignone et al. (2011) found that MLC1 protein binds the β1 subunit of the Na+/K+-ATPase and both associate in a macromolecular complex with other ion channels, including Kir4.1, AQP4, TRPV4, and cytoskeletal anchoring proteins to control osmotic balance and cell volume change in brain astrocytes during hyposmotic stress (Brignone et al., 2011; Lanciotti et al., 2012). Consistently, the intracellular MLC1− Na+/K+-ATPase interaction occurs mainly in the membrane of intracellular organelles, like early endosome-derived vacuoles that form in cultured astrocytes during hypo-osmotic shock (Brignone et al., 2011, 2014), thus further suggesting that this binding maybe relavant during osmotic stress and cell swelling. Because brain pathology in patients carrying MLC1 mutations is thought to be caused by alterations of astrocyte function in regulating osmotic balance and cell volume changes, the impairment of the MLC1− Na+/K+-ATPase complex may contribute to the astrocyte dysfunction observed in MLC disease.
Conclusions and Perspectives
Na+ dynamics are essential for regulating functional processes in glial cells. Indeed, glial Na+ signaling influences and regulates important glial activities, and plays a role in neuron-glia interaction in several neurological disorders, including cerebral ischemia, Alzheimer’s disease, epilepsy, Parkinson’s disease, Amyotrophic lateral sclerosis, and myelin diseases (Fig. 2). The emerging findings discussed in the present review have highlighted that the different glial Na+-dependent ion transporters, Na+/K+-ATPase, NCX, NKCC1, NBC, and NHE1, are critically involved in the regulation of Na+ dynamics and consequently participate in several functions including cell death and survival. More importantly, the derangement of glial ion homeostasis leads to intracellular accumulation of Na+, Ca2+, and Cl− ions in the CNS neurodegenerative diseases. These changes in turn trigger a cascade of signal transduction events which compromise glial function and culminate in cell dysfunction.
Collectively, the findings highlighted in the present review summarizes the foundation work for targeting Na+-dependent transporters in glia as a novel strategy to control important glial activities associated with intracellular Na+ derangement in different neurological disorders.
Acknowledgments
Grant sponsor: MIUR; Grant numbers: PON01_01602, PON03PE_00146_1 to L.A.; Grant sponsor: Regione Campania; Grant number: POR Campania FESR 2007–2013 MOVIE (B25C1300024007) to L.A.; Grant sponsor: Fondazione Italiana Sclerosi Multipla; Grant numbers: FISM 2012/R1 and FISM2015/R6 to F.B.; Grant sponsor: NIH; Grant numbers: RO1 NS048216 and RO1 NS38118 to D.S.; Grant sponsor: VA; Grant number: 101BX002891 to D.S.
Abbreviations
- AD
Alzheimer’s disease
- ALS
amyotrophic lateral sclerosis
- Aβ
amyloid beta
- [Ca2+]i
intracellular calcium concentrations
- CNPase
2′,3′-cyclic-nucleotide 3′-phosphodiesterase
- CNS
central nervous system
- EAE
experimental autoimmune encephalomyelitis
- ERK1/2
extracellular signal regulated kinases
- HAIR
hypoxic acidic ion-shifted Ringer
- [K+]i
extracellular potassium
- MBP
myelin basic protein
- MLC
megalencephalic leukoencephalopathy with subcortical cysts
- MN
motor neurons
- MOG
myelin oligodendrocyte glycoprotein
- MS
Multiple Sclerosis
- Na+
sodium
- NBC
Na+-HCO−3 cotransporter
- NCKX
K+-dependent Na+/Ca2+ Exchanger
- NCX
Na+/Ca2+ exchanger
- NHE
Na+/H+ exchanger
- NKA
Na+/K+ ATPase
- NKCC
Na+-K+-Cl− cotransporter
- OGD
oxygen and glucose deprivation
- OPC
oligodendrocyte precursor cell
- PD
Parkinson’s disease
- PKA
protein kinase A
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
- pMCAO
permanent middle cerebral artery occlusion
- RDP
rapid-onset dystonia Parkinsonism
- ROS
reactive oxygen species
- SOD1
superoxide dismutase 1
- VWM
vanish white matter
References
- Abdullaev IF, Rudkouskaya A, Schools GP, Kimelberg HK, Mongin AA. Pharmacological comparison of swelling-activated excitatory amino acid release and Cl− currents in cultured rat astrocytes. J Physiol. 2006;572:677–689. doi: 10.1113/jphysiol.2005.103820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato L, Boscia F, Pignataro G. Ionic transporter activity in astrocytes, microglia, and oligodendrocytes during brain ischemia. J Cereb Blood Flow Metab. 2013;33:969–982. doi: 10.1038/jcbfm.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato L, Pignataro G, Boscia F, Sirabella R, Formisano L, Saggese M, Cuomo O, Gala R, Secondo A, Viggiano D, Molinaro P, Valsecchi V, Tortiglione A, Adornetto A, Scorziello A, Cataldi M, Di Renzo GF. ncx1, ncx2, and ncx3 gene product expression and function in neuronal anoxia and brain ischemia. Ann N Y Acad Sci. 2007a;1099:413–426. doi: 10.1196/annals.1387.050. [DOI] [PubMed] [Google Scholar]
- Annunziato L, Cataldi M, Pignataro G, Secondo A, Molinaro P. Glutamate-independent calcium toxicity: introduction. Stroke. 2007b;38:661–4. doi: 10.1161/01.STR.0000247942.42349.37. [DOI] [PubMed] [Google Scholar]
- Annunziato L, Pignataro G, Di Renzo G. Pharmacology of brain Na+/Ca2+ exchanger: From molecular biology to therapeutic perspectives. Pharmacol Rev. 2004;56:633–654. doi: 10.1124/pr.56.4.5. [DOI] [PubMed] [Google Scholar]
- Aronson PS. Kinetic properties of the plasma membrane Na+-H+ exchanger. Annu Rev Physiol. 1985;47:545–560. doi: 10.1146/annurev.ph.47.030185.002553. [DOI] [PubMed] [Google Scholar]
- Banasiak KJ, Burenkova O, Haddad GG. Activation of voltage-sensitive sodium channels during oxygen deprivation leads to apoptotic neuronal death. Neuroscience. 2004;126:31–44. doi: 10.1016/S0306-4522(03)00425-1. [DOI] [PubMed] [Google Scholar]
- Bano D, Nicotera P. Ca2+ signals and neuronal death in brain ischemia. Stroke. 2007;38:674–676. doi: 10.1161/01.STR.0000256294.46009.29. [DOI] [PubMed] [Google Scholar]
- Begum G, Yuan H, Kahle KT, Li L, Wang S, Shi Y, Shmukler BE, Yang SS, Lin SH, Alper SL, Sun D. Inhibition of WNK3 kinase signaling reduces brain damage and accelerates neurological recovery after stroke. Stroke. 2015;46:1956–1965. doi: 10.1161/STROKEAHA.115.008939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benos DJ, McPherson S, Hahn BH, Chaikin MA, Benveniste EN. Cytokines and HIV envelope glycoprotein gp120 stimulate Na+/H+ exchange in astrocytes. J Biol Chem. 1994;269:13811–13816. [PubMed] [Google Scholar]
- Bernardinelli Y, Azarias G, Chatton JY. In situ fluorescence imaging of glutamate-evoked mitochondrial Na+ responses in astrocytes. Glia. 2006;54:460–470. doi: 10.1002/glia.20387. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Calcium signalling and cell proliferation. Bioessays. 1995;17:491–500. doi: 10.1002/bies.950170605. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Juhaszova M, Golovina VA, Church PJ, Stanley EF. Na/Ca exchanger and PMCA localization in neurons and astrocytes: Functional implications. Ann N Y Acad Sci. 2002;976:356–366. doi: 10.1111/j.1749-6632.2002.tb04762.x. [DOI] [PubMed] [Google Scholar]
- Bondarenko A, Chesler M. Rapid astrocyte death induced by transient hypoxia, acidosis, and extracellular ion shifts. Glia. 2001;34:134–142. doi: 10.1002/glia.1048. [DOI] [PubMed] [Google Scholar]
- Bondarenko AI, Jean-Quartier C, Parichatikanond W, Alam MR, Waldeck-Weiermair M, Malli R, Graier WF. Mitochondrial Ca(2+) uniporter (MCU)-dependent and MCU-independent Ca(2+) channels coexist in the inner mitochondrial membrane. Pflugers Arch. 2014;466:1411–1420. doi: 10.1007/s00424-013-1383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarenko A, Svichar N, Chesler M. Role of Na+-H+ and Na+-Ca2+ exchange in hypoxia-related acute astrocyte death. Glia. 2005;49:143–152. doi: 10.1002/glia.20107. [DOI] [PubMed] [Google Scholar]
- Boor PK, de Groot K, Waisfisz Q, Kamphorst W, Oudejans CB, Powers JM, Pronk JC, Scheper GC, van der Knaap MS. MLC1: A novel protein in distal astroglial processes. J Neuropathol Exp Neurol. 2005;64:412–419. doi: 10.1093/jnen/64.5.412. [DOI] [PubMed] [Google Scholar]
- Boscia F, Casamassa A, Secondo A, Esposito A, Pannaccione A, Sirabella R, Pignataro G, Cuomo O, Vinciguerra A, de Rosa V, Annunziato L. NCX1 exchanger cooperates with calretinin to confer preconditioning-induced tolerance against cerebral ischemia in the striatum. Mol Neurobiol. 2016;53:1365–1376. doi: 10.1007/s12035-015-9095-4. [DOI] [PubMed] [Google Scholar]
- Boscia F, D’Avanzo C, Pannaccione A, Secondo A, Casamassa A, Formisano L, Guida N, Scorziello A, Di Renzo G, Annunziato L. New roles of NCX in glial cells: Activation of microglia in ischemia and differentiation of oligodendrocytes. Adv Exp Med Biol. 2013;961:307–316. doi: 10.1007/978-1-4614-4756-6_26. [DOI] [PubMed] [Google Scholar]
- Boscia F, D’Avanzo C, Pannaccione A, Secondo A, Casamassa A, Formisano L, Guida N, Sokolow S, Herchuelz A, Annunziato L. Silencing or knocking out the Na(+)/Ca(2+) exchanger-3 (NCX3) impairs oligodendrocyte differentiation. Cell Death Differ. 2012;19:562–572. doi: 10.1038/cdd.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscia F, Gala R, Pannaccione A, Secondo A, Scorziello A, Di Renzo G, Annunziato L. NCX1 expression and functional activity increase in microglia invading the infarct core. Stroke. 2009;40:3608–3617. doi: 10.1161/STROKEAHA.109.557439. [DOI] [PubMed] [Google Scholar]
- Boscia F, Gala R, Pignataro G, de Bartolomeis A, Cicale M, Ambesi-Impiombato A, Di Renzo G, Annunziato L. Permanent focal brain ischemia induces isoform-dependent changes in the pattern of Na+/Ca2+ exchanger gene expression in the ischemic core, periinfarct area, and intact brain regions. J Cereb Blood Flow Metab. 2006;26:502–517. doi: 10.1038/sj.jcbfm.9600207. [DOI] [PubMed] [Google Scholar]
- Boussouf A, Lambert RC, Gaillard S. Voltage-dependent Na+-HCO3− cotransporter and Na+/H+ exchanger are involved in intracellular pH regulation of cultured mature rat cerebellar oligodendrocytes. Glia. 1997;19:74–84. doi: 10.1002/(sici)1098-1136(199701)19:1<74::aid-glia8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Brignone MS, Lanciotti A, Macioce P, Macchia G, Gaetani M, Aloisi F, Petrucci TC, Ambrosini E. The beta1 subunit of the Na, K-ATPase pump interacts with megalencephalic leucoencephalopathy with subcortical cysts protein 1 (MLC1) in brain astrocytes: New insights into MLC pathogenesis. Hum Mol Genet. 2011;20:90–103. doi: 10.1093/hmg/ddq435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignone MS, Lanciotti A, Visentin S, De Nuccio C, Molinari P, Camerini S, Diociaiuti M, Petrini S, Minnone G, Crescenzi M, Laudiero LB, Bertini E, Petrucci TC, Ambrosini E. Megalencephalic leukoencephalopathy with subcortical cysts protein-1 modulates endosomal pH and protein trafficking in astrocytes: Relevance to MLC disease pathogenesis. Neurobiol Dis. 2014;66:1–18. doi: 10.1016/j.nbd.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- Campbell BC, McLean CA, Culvenor JG, Gai WP, Blumbergs PC, Jäkälä P, Beyreuther K, Masters CL, Li QX. The solubility of α-synuclein in multiple system atrophy differs from that of dementia with Lewy bodies and Parkinson’s disease. J Neurochem. 2001;76:87–96. doi: 10.1046/j.1471-4159.2001.00021.x. [DOI] [PubMed] [Google Scholar]
- Cannon SC. Paying the price at the pump: Dystonia from mutations in a Na+/K+-ATPase. Neuron. 2004;43:153–154. doi: 10.1016/j.neuron.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Carmignoto G, Haydon PG. Astrocyte calcium signaling and epilepsy. Glia. 2012;60:1227–1233. doi: 10.1002/glia.22318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamassa A, La Rocca C, Sokolow S, Herchuelz A, Matarese G, Annunziato L, Boscia F. Ncx3 gene ablation impairs oligodendrocyte precursor response and increases susceptibility to experimental autoimmune encephalomyelitis. Glia. 2016;64:1124–1137. doi: 10.1002/glia.22985. [DOI] [PubMed] [Google Scholar]
- Cengiz P, Kintner DB, Chanana V, Yuan H, Akture E, Kendigelen P, Begum G, Fidan E, Uluc K, Ferrazzano P, Sun D. Sustained Na+/H+ exchanger activation promotes gliotransmitter release from reactive hippocampal astrocytes following oxygen-glucose deprivation. PLoS One. 2014;9:e84294. doi: 10.1371/journal.pone.0084294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan NB, Lee JM, Siegel GJ. Na, K-ATPase mRNA levels and plaque load in Alzheimer’s disease. J Mol Neurosci. 1997;9:151–166. doi: 10.1007/BF02800498. [DOI] [PubMed] [Google Scholar]
- Chen H, Kintner DB, Jones M, Matsuda T, Baba A, Kiedrowski L. AMPA-mediated excitotoxicity in oligodendrocytes: role for Na(+)-K(+)-Cl(−) cotransport and reversal of Na(+)/Ca(2+) exchanger. J Neurochem. 2007;102:1783–1795. doi: 10.1111/j.1471-4159.2007.04638.x. [DOI] [PubMed] [Google Scholar]
- Chen H, Luo J, Kintner DB, Shull GE, Sun D. Na(+)-dependent chloride transporter (NKCC1)-null mice exhibit less gray and white matter damage after focal cerebral ischemia. J Cereb Blood Flow Metab. 2005;25:54–66. doi: 10.1038/sj.jcbfm.9600006. [DOI] [PubMed] [Google Scholar]
- Cholet N, Pellerin L, Magistretti PJ, Hamel E. Similar perisynaptic glial localization for the Na+, K+-ATPase alpha 2 subunit and the glutamate transporters GLAST and GLT-1 in the rat somatosensory cortex. Cereb Cortex. 2002;12:515–525. doi: 10.1093/cercor/12.5.515. [DOI] [PubMed] [Google Scholar]
- Chong SY, Chan JR. Tapping into the glial reservoir: Cells committed to remaining uncommitted. J Cell Biol. 2010;188:305–312. doi: 10.1083/jcb.200905111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Welsh CA, Barres BA, Stevens B. Do glia drive synaptic and cognitive impairment in disease? Nat Neurosci. 2015;18:1539–1545. doi: 10.1038/nn.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary RT, Sun H, Huynh T, Manning SM, Li Y, Rotenberg A, Talos DM, Kahle KT, Jackson M, Rakhade SN, Berry G, Jensen FE. Bumetanide enhances phenobarbital efficacy in a rat model of hypoxic neonatal seizures. PLoS One. 2013;8:e57148. doi: 10.1371/journal.pone.0057148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Cooper DS, Yang HS, He P, Kim E, Rajbhandari I, Yun CC, Choi I. Sodium/bicarbonate cotransporter NBCn1/slc4a7 increases cytotoxicity in magnesium depletion in primary cultures of hippocampal neurons. Eur J Neurosci. 2009;29:437–446. doi: 10.1111/j.1460-9568.2008.06611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero-Erausquin M, Coull JA, Boudreau D, Rolland M, De Koninck Y. Differential maturation of GABA action and anion reversal potential in spinal lamina I neurons: Impact of chloride extrusion capacity. J Neurosci. 2005;25:9613–9623. doi: 10.1523/JNEUROSCI.1488-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Coulter DA, Steinhäuser C. Role of astrocytes in epilepsy. Cold Spring Harbor Perspect Med. 2015;5:a022434. doi: 10.1101/cshperspect.a022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damier P, Hammond C, Ben-Ari Y. Bumetanide to treat Parkinson disease: A report of 4 cases. Clin Neuropharmacol. 2016;39:57–59. doi: 10.1097/WNF.0000000000000114. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prod Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390:680–683. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- de Carvalho AP, Sweadner KJ, Penniston JT, Zaremba J, Liu L, Caton M, Linazasoro G, Borg M, Tijssen MA, Bressman SB, Dobyns WB, Brashear A, Ozelius LJ. Mutations in the Na+/K+-ATPase alpha3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. Neuron. 2004;43:169–175. doi: 10.1016/j.neuron.2004.06.028. [DOI] [PubMed] [Google Scholar]
- De Keyser J, Mostert JP, Koch MW. Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders. J Neurol Sci. 2008;267:3–16. doi: 10.1016/j.jns.2007.08.044. [DOI] [PubMed] [Google Scholar]
- Desai MK, Mastrangelo MA, Ryan DA, Sudol KL, Narrow WC, Bowers WJ. Early oligodendrocyte/myelin pathology in Alzheimer’s disease mice constitutes a novel therapeutic target. Am J Pathol. 2010;177:1422–1435. doi: 10.2353/ajpath.2010.100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CA, Gordon MN, Wilcock DM, Herber DL, Freeman MJ, Morgan D. Dysregulation of Na+/K+ ATPase by amyloid in APP+PS1 transgenic mice. BMC Neurosci. 2005;6:7. doi: 10.1186/1471-2202-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008;3:637–648. doi: 10.1016/j.stem.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Dobretsov M, Stimers JR. Characterization of the Na/K pump current in N20.1 oligodendrocytes. Brain Res. 1996;724:103–111. doi: 10.1016/0006-8993(96)00171-0. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- Ernest NJ, Sontheimer H. Extracellular glutamine is a critical modulator for regulatory volume increase in human glioma cells. Brain Res. 2007;1144:231–238. doi: 10.1016/j.brainres.2007.01.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faff L, Ohlemeyer C, Kettenmann H. Intracellular pH regulation in cultured microglial cells from mouse brain. J Neurosci Res. 1996;46:294–304. doi: 10.1002/(SICI)1097-4547(19961101)46:3<294::AID-JNR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Fern R, Ransom BR, Waxman SG. Voltage-gated calcium channels in CNS white matter: Role in anoxic injury. J Neurophysiol. 1995;74:369–377. doi: 10.1152/jn.1995.74.1.369. [DOI] [PubMed] [Google Scholar]
- Ferraiuolo L, Higginbottom A, Heath PR, Barber S, Greenald D, Kirby J, Shaw PJ. Dysregulation of astrocyte-motoneuron cross-talk in mutant superoxide dismutase 1-related amyotrophic lateral sclerosis. Brain. 2011;134:2627–2641. doi: 10.1093/brain/awr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. A new mechanism of nervous system plasticity: Activity-dependent myelination. Nat Rev Neurosci. 2015;16:756–767. doi: 10.1038/nrn4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink D, Knapp PE, Mata M. Differential expression of Na, K-ATPase isoforms in oligodendrocytes and astrocytes. Dev Neurosci. 1996;18:319–326. doi: 10.1159/000111422. [DOI] [PubMed] [Google Scholar]
- Floyd CL, Gorin FA, Lyeth BG. Mechanical strain injury increases intracellular sodium and reverses Na+/Ca2+ exchange in cortical astrocytes. Glia. 2005;51:35–46. doi: 10.1002/glia.20183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo G, Barowski J, Ravits J, Siddique T, Lingrel JB, Robertson J, Steen H, Bonni A. An α2-Na/K ATPase/α-adducin complex in astrocytes triggers non-cell autonomous neurodegeneration. Nat Neurosci. 2014;17:1710–1719. doi: 10.1038/nn.3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao K, Wang CR, Jiang F, Wong AY, Su N, Jiang JH, Chai RC, Vatcher G, Teng J, Chen J, Jiang YW, Yu AC. Traumatic scratch injury in astrocytes triggers calcium influx to activate the JNK/c-Jun/AP-1 pathway and switch on GFAP expression. Glia. 2013;61:2063–2077. doi: 10.1002/glia.22577. [DOI] [PubMed] [Google Scholar]
- Giatti S, Boraso M, Abbiati F, Ballarini E, Calabrese D, Santos-Galindo M, Rigolio R, Pesaresi M, Caruso D, Viviani B, Cavaletti G, Garcia-Segura LM, Melcangi RC. Multimodal analysis in acute and chronic experimental autoimmune encephalomyelitis. J Neuroimmune Pharmacol. 2013;8:238–250. doi: 10.1007/s11481-012-9385-9. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan G, Awasthi A, Belkaid W, De Faria OJ, Liazoghli D, Colman DR, Dhaunchak AS. Lipidome and proteome map of myelin membranes. J Neurosci Res. 2013;91:321–334. doi: 10.1002/jnr.23157. [DOI] [PubMed] [Google Scholar]
- Hattori N, Kitagawa K, Higashida T, Yagyu K, Shimohama S, Wataya T, Perry G, Smith MA, Inagaki C. CI−-ATPase and Na+/K+-ATPase activities in Alzheimer’s disease. Neurosci Lett. 1998;254:141–144. doi: 10.1016/s0304-3940(98)00654-5. [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Kann O, Ohlemeyer C, Hanisch UK, Kettenmann H. Elevation of basal intracellular calcium as a central element in the activation of brain macrophages (microglia): Suppression of receptor-evoked calcium signaling and control of release function. J Neurosci. 2003;23:4410–4419. doi: 10.1523/JNEUROSCI.23-11-04410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden CP, Haughey NJ, Nath A, Geiger JD. Role of Na+/H+ exchangers, excitatory amino acid receptors and voltage operated Ca2+ channels in human immunodeficiency virus type 1 gp120-mediated increases in intracellular Ca2+ in human neurons and astrocytes. Neuroscience. 1999;91:1369–1378. doi: 10.1016/s0306-4522(98)00714-3. [DOI] [PubMed] [Google Scholar]
- Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, Lemere CA, Selkoe DJ, Stevens B. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, Chen J. Microglial and macrophage polarization—New prospects for brain repair. Nat Rev Neurol. 2015;11:56–64. doi: 10.1038/nrneurol.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang IK, Yoo KY, An SJ, Li H, Lee CH, Choi JH, Lee JY, Lee BH, Kim YM, Kwon YG, Won MH. Late expression of Na+/H+ exchanger 1 (NHE1) and neuroprotective effects of NHE inhibitor in the gerbil hippocampal CA1 region induced by transient ischemia. Exp Neurol. 2008;212:314–323. doi: 10.1016/j.expneurol.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Ibarreta D, Parrilla R, Ayuso MS. Altered Ca2+ homeostasis in lymphoblasts from late-onset Alzheimer’s disease patients. Alzheimer Dis Assoc Disord. 1997;11:220–227. [PubMed] [Google Scholar]
- Ibarreta D, Urcelay E, Parrilla R, Ayuso MS. Distinct pH homeostatic features in lymphoblasts from Alzheimer’s disease patients. Ann Neurol. 1998;44:216–222. doi: 10.1002/ana.410440212. [DOI] [PubMed] [Google Scholar]
- Ifuku M, Farber K, Okuno Y, Yamakawa Y, Miyamoto T, Nolte C. Bradykinin-induced microglial migration mediated by B1-bradykinin receptors depends on Ca2+ influx via reverse-mode activity of the Na+/Ca2+ exchanger. J Neurosci. 2007;27:13065–13073. doi: 10.1523/JNEUROSCI.3467-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Onimaru H, Yamada J, Inoue K, Ueno S, Onaka T, Toyoda H, Arata A, Ishikawa TO, Taketo MM, Fukuda A, Kawakami K. Malfunction of respiratory-related neuronal activity in Na+, K+-ATPase alpha2 subunit-deficient mice is attributable to abnormal Cl− homeostasis in brainstem neurons. J Neurosci. 2004;24:10693–10701. doi: 10.1523/JNEUROSCI.2909-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzie LL, Hu MY, Park HK, Jackson MC, Yu J, Maxwell JR, Jensen FE. Chloride cotransporter NKCC1 inhibitor bumetanide protects against white matter injury in a rodent model of periventricular leukomalacia. Pediatr Res. 2015;77:554–562. doi: 10.1038/pr.2015.9. [DOI] [PubMed] [Google Scholar]
- Jayakumar AR, Bethea JR, Tong XY, Gomez J, Norenberg MD. NF-κB in the mechanism of brain edema in acute liver failure: Studies in transgenic mice. Neurobiol Dis. 2011;41:498–507. doi: 10.1016/j.nbd.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar AR, Liu M, Moriyama M, Ramakrishnan R, Forbush B, III, Reddy PV, Norenberg MD. Na–K–Cl Cotransporter-1 in the mechanism of ammonia-induced astrocyte swelling. J Biol Chem. 2008;283:33874–33882. doi: 10.1074/jbc.M804016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon D, Chu K, Jung KH, Kim M, Yoon BW, Lee CJ, Oh U, Shin HS. Na(+)/Ca(2+) exchanger 2 is neuroprotective by exporting Ca(2+) during a transient focal cerebral ischemia in the mouse. Cell Calcium. 2008;43:482–491. doi: 10.1016/j.ceca.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Jung YW, Choi IJ, Kwon TH. Altered expression of sodium transporters in ischemic penumbra after focal cerebral ischemia in rats. Neurosci Res. 2007;59:152–159. doi: 10.1016/j.neures.2007.06.1470. [DOI] [PubMed] [Google Scholar]
- Kahle KT, Simard JM, Staley KJ, Nahed BV, Jones PS, Sun D. Molecular mechanisms of ischemic cerebral edema: Role of electroneutral ion transport. Physiology (Bethesda) 2009;24:257–265. doi: 10.1152/physiol.00015.2009. [DOI] [PubMed] [Google Scholar]
- Kahle KT, Staley KJ, Nahed BV, Gamba G, Hebert SC, Lifton RP, Mount DB. Roles of the cation-chloride cotransporters in neurological disease. Nat Clin Pract Neurol. 2008;4:490–503. doi: 10.1038/ncpneuro0883. [DOI] [PubMed] [Google Scholar]
- Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J. Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat Rev Neurosci. 2014;15:637–654. doi: 10.1038/nrn3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang TC, An SJ, Park SK, Hwang IK, Bae JC, Suh JG, Oh JS, Won MH. Changes in Na+–K+–Cl− cotransporter immunore-activity in the gerbil hippocampus following transient ischemia. Neurosci Res. 2002;44:249–254. doi: 10.1016/s0168-0102(02)00131-1. [DOI] [PubMed] [Google Scholar]
- Kaplan JH. Biochemistry of Na, K-ATPase. Annu Rev Biochem. 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- Karus C, Mondragão MA, Ziemens D, Rose CR. Astrocytes restrict discharge duration and neuronal sodium loads during recurrent network activity. Glia. 2015;63:936–957. doi: 10.1002/glia.22793. [DOI] [PubMed] [Google Scholar]
- Kersh AE, Hartzler LK, Havlin K, Hubbell BB, Nanagas V, Kalra A, Chua J, Whitesell R, Ritucci NA, Dean JB, Putnam RW. pH regulating transporters in neurons from various chemosensitive brainstem regions in neonatal rats. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1409–R1420. doi: 10.1152/ajpregu.91038.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Schlue WR. Intracellular pH regulation in cultured mouse oligodendrocytes. J Physiol. 1988;406:147–162. doi: 10.1113/jphysiol.1988.sp017373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachaturian ZS. Calcium regulations: Final common pathway for age associated neurodegenerative processes. Neurosci Res Commun. 1993;13:s3–s6. [Google Scholar]
- Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci. 2015;18:942–952. doi: 10.1038/nn.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintner DB, Look A, Shull GE, Sun D. Stimulation of astrocyte Na+/H+ exchange activity in response to in vitro ischemia depends in part on activation of ERK1/2. Am J Physiol Cell Physiol. 2005;289:C934–C945. doi: 10.1152/ajpcell.00092.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintner DB, Luo J, Gerdts J, Ballard AJ, Shull GE, Sun D. Role of Na+-K+-Cl− cotransport and Na+/Ca2+ exchange in mitochondrial dysfunction in astrocytes following in vitro ischemia. Am J Physiol Cell Physiol. 2007;292:C1113–C1122. doi: 10.1152/ajpcell.00412.2006. [DOI] [PubMed] [Google Scholar]
- Kintner DB, Su G, Lenart B, Ballard AJ, Meyer JW, Ng LL, Shull GE, Sun D. Increased tolerance to oxygen and glucose deprivation in astrocytes from Na(+)/H(+) exchanger isoform 1 nullmice. Am J Physiol Cell Physiol. 2004;287:C12–C21. doi: 10.1152/ajpcell.00560.2003. [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Kettenmann H, Verkhratsky A. Na+/Ca2+ exchanger modulates kainate-triggered Ca2+ signaling in Bergmann glial cells in situ. Faseb J. 1997;11:566–572. doi: 10.1096/fasebj.11.7.9212080. [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Parpura V, Verkhratsky A. Sodium dynamics: Another key to astroglial excitability? Trends Neurosci. 2012;35:497–506. doi: 10.1016/j.tins.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Kristensen K, Berenbrink M, Koldkjaer P, Abe A, Wang T. Minimal volume regulation after shrinkage of red blood cells from five species of reptiles. Comp Biochem Physiol A Mol Integr Physiol. 2008;150:46–51. doi: 10.1016/j.cbpa.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, DeStafano AL, Bis JC, Beecham GW, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45:1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti A, Brignone MS, Bertini E, Petrucci TC, Aloisi F, Ambrosini E. Astrocytes: Emerging stars in leukodystrophy pathogenesis. Transl Neurosci. 2013:4. doi: 10.2478/s13380-013-0118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti A, Brignone MS, Molinari P, Visentin S, De Nuccio C, Macchia G, Aiello C, Bertini E, Aloisi F, Petrucci TC, Ambrosini E. Megalencephalic leukoencephalopathy with subcortical cysts protein 1 functionally cooperates with the TRPV4 cation channel to activate the response of astrocytes to osmotic stress: Dysregulation by pathological mutations. Hum Mol Genet. 2012;21:2166–2180. doi: 10.1093/hmg/dds032. [DOI] [PubMed] [Google Scholar]
- Langer J, Rose CR. Synaptically induced sodium signals in hippocampal astrocytes in situ. J Physiol. 2009;587:5859–5877. doi: 10.1113/jphysiol.2009.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascola C, Kraig RP. Astroglial acid-base dynamics in hyperglycemic and normoglycemic global ischemia. Neurosci Biobehav Rev. 1997;21:143–150. doi: 10.1016/s0149-7634(96)00004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis JA, Bekar LK, Walz W. Potassium homeostasis in the ischemic brain. Glia. 2005;50:407–416. doi: 10.1002/glia.20145. [DOI] [PubMed] [Google Scholar]
- Lenart B, Kintner DB, Shull GE, Sun D. Na-K-Cl cotransporter-mediated intracellular Na+ accumulation affects Ca2+ signaling in astrocytes in an in vitro ischemic model. J Neurosci. 2004;24:9585–9597. doi: 10.1523/JNEUROSCI.2569-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng T, Shi Y, Xiong ZG, Sun D. Proton-sensitive cation channels and ion exchangers in ischemic brain injury: New therapeutic targets for stroke? Prog Neurobiol. 2014;115:189–209. doi: 10.1016/j.pneurobio.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Harms KM, Ventura PB, Lagace DC, Eisch AJ, Cunningham LA. Focal cerebral ischemia induces a multilineage cytogenic response from adult subventricular zone that is predominantly gliogenic. Glia. 2010;58:1610–1619. doi: 10.1002/glia.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Jiang Q, Stys PK. Important role of reverse Na(+)-Ca(2+) exchange in spinal cord white matter injury at physiological temperature. J Neurophysiol. 2000;84:1116–1119. doi: 10.1152/jn.2000.84.2.1116. [DOI] [PubMed] [Google Scholar]
- Liang D, Bhatta S, Gerzanich V, Simard JM. Cytotoxic edema: Mechanisms of pathological cell swelling. Neurosurg Focus. 2007;22:E2. doi: 10.3171/foc.2007.22.5.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguri G, Taddei N, Nassi P, Latorraca S, Nediani C, Sorbi S. Changes in Na+, K+-ATPase, Ca2+-ATPase and some soluble enzymes related to energy metabolism in brains of patients with Alzheimer’s disease. Neurosci Lett. 1990;112:338–342. doi: 10.1016/0304-3940(90)90227-z. [DOI] [PubMed] [Google Scholar]
- Liu Y, Kintner DB, Chanana V, Algharabli J, Chen X, Gao Y, Chen J, Ferrazzano P, Olson JK, Sun D. Activation of microglia depends on Na+/H+ exchange-mediated H+ homeostasis. J Neurosci. 2010;30:15210–15220. doi: 10.1523/JNEUROSCI.3950-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longuemare MC, Rose CR, Farrell K, Ransom BR, Waxman SG, Swanson RA. K+-induced reversal of astrocyte glutamate uptake is limited by compensatory changes in intracellular Na+ Neuroscience. 1999;93:285–292. doi: 10.1016/s0306-4522(99)00152-9. [DOI] [PubMed] [Google Scholar]
- Lu KT, Wu CY, Cheng NC, Wo YY, Yang JT, Yen HH, Yang YL. Inhibition of the Na+-K+-2Cl−-cotransporter in choroid plexus attenuates traumatic brain injury-induced brain edema and neuronal damage. Eur J Pharmacol. 2006;548:99–105. doi: 10.1016/j.ejphar.2006.07.048. [DOI] [PubMed] [Google Scholar]
- Luo J, Chen H, Kintner DB, Shull GE, Sun D. Decreased neuronal death in Na+/H+exchanger isoform 1-null mice after in vitro and in vivo ischemia. J Neurosci. 2005;25:11256–11268. doi: 10.1523/JNEUROSCI.3271-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Sun D. Physiology and pathophysiology of Na(+)/H(+) exchange isoform 1 in the central nervous system. Curr Neurovasc Res. 2007;4:205–215. doi: 10.2174/156720207781387178. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism. Relevance to functional brain imaging and to neurodegenerative disorders. Ann N Y Acad Sci. 1996;777:380–387. doi: 10.1111/j.1749-6632.1996.tb34449.x. [DOI] [PubMed] [Google Scholar]
- Majumdar D, Bevensee MO. Na-coupled bicarbonate transporters of the solute carrier 4 family in the nervous system: Function, localization, and relevance to neurologic function. Neuroscience. 2010;171:951–972. doi: 10.1016/j.neuroscience.2010.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malarkey EB, Parpura V. Mechanisms of glutamate release from astrocytes. Neurochem Int. 2008;52:142–154. doi: 10.1016/j.neuint.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maly K, Strese K, Kampfer S, Ueberall F, Baier G, Ghaffari-Tabrizi N, Grunicke HH, Leitges M. Critical role of protein kinase C alpha and calcium in growth factor induced activation of the Na(+)/H(+) exchanger NHE1. FEBS Lett. 2002;521:205–210. doi: 10.1016/s0014-5793(02)02867-3. [DOI] [PubMed] [Google Scholar]
- Manhas N, Shi Y, Taunton J, Sun D. p90RSK activation contributes to cerebral ischemic damage via phosphorylation of Na+/H+ exchanger isoform 1. J Neurochem. 2010;114:1476–1486. doi: 10.1111/j.1471-4159.2010.06868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetto MC, Muotri AR, Mu Y, Smith AM, Cezar GG, Gage FH. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3:649–657. doi: 10.1016/j.stem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Martin-Vasallo P, Wetzel RK, Garcia-Segura LM, Molina-Holgado E, Arystarkhova E, Sweadner KJ. Oligodendrocytes in brain and optic nerve express the beta3 subunit isoform of Na, K-ATPase. Glia. 2000;31:206–218. doi: 10.1002/1098-1136(200009)31:3<206::aid-glia20>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Arakawa N, Takuma K, Kishida Y, Kawasaki Y, Sakaue M. SEA0400, a novel and selective inhibitor of the Na+-Ca2+ exchanger, attenuates reperfusion injury in the in vitro and in vivo cerebral ischemic models. J Pharmacol Exp Ther. 2001;298:249–256. [PubMed] [Google Scholar]
- Matsuda T, Nagano T, Takemura M, Baba A. Topics on the Na+/Ca2+ exchanger: Responses of Na+/Ca2+ exchanger to interferon-gamma and nitric oxide in cultured microglia. J Pharmacol Sci. 2006;102:22–26. doi: 10.1254/jphs.fmj06002x4. [DOI] [PubMed] [Google Scholar]
- Melom JE, Littleton JT. Mutation of a NCKX eliminates glial microdomain calcium oscillations and enhances seizure susceptibility. J Neurosci. 2013;33:1169–1178. doi: 10.1523/JNEUROSCI.3920-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minelli A, Castaldo P, Gobbi P, Salucci S, Magi S, Amoroso S. Cellular and subcellular localization of Na+-Ca2+ exchanger protein isoforms, NCX1, NCX2, and NCX3 in cerebral cortex and hippocampus of adult rat. Cell Calcium. 2007;41:221–234. doi: 10.1016/j.ceca.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin RJ, ffrench-Constant C. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistur R, Mosconi L, Santi SD, Guzman M, Li Y, Tsui W, de Leon MJ. Current challenges for the early detection of Alzheimer’s disease: Brain imaging and CSF studies. J Clin Neurol. 2009;5:153–166. doi: 10.3988/jcn.2009.5.4.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinaro P, Cuomo O, Pignataro G, Boscia F, Sirabella R, Pannaccione A, Secondo A, Scorziello A, Adornetto A, Gala R, Viggiano D, Sokolow S, Herchuelz A, Schurmans S, Di Renzo G, Annunziato L. Targeted disruption of Na+/Ca2+ exchanger 3 (NCX3) gene leads to a worsening of ischemic brain damage. J Neurosci. 2008;28:1179–1184. doi: 10.1523/JNEUROSCI.4671-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinaro P, Sirabella R, Pignataro G, Petrozziello T, Secondo A, Boscia F, Vinciguerra A, Cuomo O, Philipson KD, De Felice MD, Lauro R, Di Renzo G, Annunziato L. Neuronal NCX1 overexpression induces stroke resistance while knockout induces vulnerability via Akt. J Cereb Blood Flow Metab. 2015 doi: 10.1177/0271678X15611913. pii: 0271678X15611913. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Pupi A, De Leon MJ. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s disease. Ann N Y Acad Sci. 2008;1147:180–195. doi: 10.1196/annals.1427.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley AE, Williams MT, Schaefer TL, Bohanan CS, Neumann JC, Behbehani MM, Vorhees CV, Lingrel JB. Deficiency in Na, K-ATPase alpha isoform genes alters spatial learning, motor activity, and anxiety in mice. J Neurosci. 2007;27:616–626. doi: 10.1523/JNEUROSCI.4464-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano T, Kawasaki Y, Baba A, Takemura M, Matsuda T. Upregulation of Na(+)-Ca2+ exchange activity by interferon-gamma in cultured rat microglia. J Neurochem. 2004;90:784–791. doi: 10.1111/j.1471-4159.2004.02511.x. [DOI] [PubMed] [Google Scholar]
- Nash B, Thomson CE, Linington C, Arthur AT, McClure JD, McBride MW, Barnett SC. Functional duality of astrocytes in myelination. J Neurosci. 2011;31:13028–13038. doi: 10.1523/JNEUROSCI.1449-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave KA. Oligodendrocytes and the “micro brake” of progenitor cell proliferation. Neuron. 2010;65:577–579. doi: 10.1016/j.neuron.2010.02.026. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Verkhratsky A. Artifact versus reality—How astrocytes contribute to synaptic events. Glia. 2012;60:1013–1023. doi: 10.1002/glia.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepomuceno R, Sun D. Pharmacological inhibition of cation-chloride cotransporters for neurological diseases. Neural Regen Res. 2015;10:1924–1925. doi: 10.4103/1673-5374.172313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell EW, Stanley EF, Schlichter LC. Reversed Na+/Ca2+ exchange contributes to Ca2+ influx and respiratory burst in microglia. Channels (Austin) 2007;1:366–376. doi: 10.4161/chan.5391. [DOI] [PubMed] [Google Scholar]
- Nilsen LH, Witter MP, Sonnewald U. Neuronal and astrocytic metabolism in a transgenic rat model of Alzheimer’s disease. J Cereb Blood Flow Metab. 2014;34:906–914. doi: 10.1038/jcbfm.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Muccigrosso MM, Godbout JP. Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology. 2015;96:29–41. doi: 10.1016/j.neuropharm.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norenberg MD. The role of astrocytes in hepatic encephalopathy. Neurochem Pathol. 1987;6:13–33. doi: 10.1007/BF02833599. [DOI] [PubMed] [Google Scholar]
- O’Donnell ME, Duong V, Suvatne J, Foroutan S, Johnson DM. Arginine vasopressin stimulation of cerebral microvascular endothelial cell Na-K-Cl cotransport activity is V1 receptor- and [Ca]-dependent. Am J Physiol. 2005;289:C283–C292. doi: 10.1152/ajpcell.00001.2005. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Yanazawa M, Sasahara T, Kitamura Y, Hiroaki H, Fukazawa Y, Kii I, Nishiyama T, Kakita A, Takeda H, Takeuchi A, Arai Y, Ito A, Komura H, Hirao H, Satomura K, Inoue M, Muramatsu S, Matsui K, Tada M, Sato M, Saijo E, Shigemitsu Y, Sakai S, Umetsu Y, Goda N, Takino N, Takahashi H, Hagiwara M, Sawasaki T, Iwasaki G, Nakamura Y, Nabeshima Y, Teplow DB, Hoshi M. Na, K-ATPase α3 is a death target of Alzheimer patient amyloid-β assembly. Proc Natl Acad Sci USA. 2015;112:E4465–E4474. doi: 10.1073/pnas.1421182112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski J, Grinstein S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Arch. 2004;447:549–565. doi: 10.1007/s00424-003-1110-3. [DOI] [PubMed] [Google Scholar]
- Ostby I, Oyehaug L, Einevoll GT, Nagelhus EA, Plahte E, Zeuthen T, Lloyd CM, Ottersen OP, Omholt SW. Astrocytic mechanisms explaining neural-activity-induced shrinkage of extraneuronal space. PLoS Comput Biol. 2009;5:e1000272. doi: 10.1371/journal.pcbi.1000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otvos L, Jr, Szendrei GI, Lee VM, Mantsch HH. Human and rodent Alzheimer beta-amyloid peptides acquire distinct conformations in membrane-mimicking solvents. Eur J Biochem. 1993;1:249–257. doi: 10.1111/j.1432-1033.1993.tb19893.x. [DOI] [PubMed] [Google Scholar]
- Paluzzi S, Alloisio S, Zappettini S, Milanese M, Raiteri L, Nobile M, Bonanno G. Adult astroglia is competent for Na+/Ca2+ exchanger-operated exocytotic glutamate release triggered by mild depolarization. J Neurochem. 2007;103:1196–1207. doi: 10.1111/j.1471-4159.2007.04826.x. [DOI] [PubMed] [Google Scholar]
- Pannaccione A, Secondo A, Molinaro P, D’Avanzo C, Cantile M, Esposito A, Boscia F, Scorziello A, Sirabella R, Sokolow S, Herchuelz A, Di Renzo G, Annunziato L. A new concept: Aβ1–42 generates a hyperfunctional proteolytic NCX3 fragment that delays caspase-12 activation and neuronal death. J Neurosci. 2012;32:10609–10617. doi: 10.1523/JNEUROSCI.6429-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27:1641–1647. doi: 10.1161/01.str.27.9.1641. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Papa M, Canitano A, Boscia F, Castaldo P, Sellitti S, Porzig H, Taglialatela M, Annunziato L. Differential expression of the Na+-Ca2+ exchanger transcripts and proteins in rat brain regions. J Comp Neurol. 2003;461:31–48. doi: 10.1002/cne.10665. [DOI] [PubMed] [Google Scholar]
- Pappalardo LW, Black JA, Waxman SG. Sodium channels in astroglia and microglia. Glia. 2016 doi: 10.1002/glia.22967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappalardo LW, Samad OA, Black JA, Waxman SG. Voltage-gated sodium channel Nav 1.5 contributes to astrogliosis in an in vitro model of glial injury via reverse Na+/Ca2+ exchange. Glia. 2014;62:1162–1175. doi: 10.1002/glia.22671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris S, Pouysstgur J. Growth factors activate the Na+/H+ antiporter in quiescent fibroblasts by increasing its affinity for intracellular H+ J Biol Chem. 1984;259:10989–10994. [PubMed] [Google Scholar]
- Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: A review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21:754–774. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- Parpura V, Verkhratsky A. Homeostatic function of astrocytes: Ca(2+) and Na(+) signalling. Transl Neurosci. 2012;3:334–344. doi: 10.2478/s13380-012-0040-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SF, O’Donnell ME, Anderson SE, Cala PM. Physiology and pathophysiology of Na+/H+ exchange and Na+-K+–2Cl− cotransport in the heart, brain, and blood. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1–25. doi: 10.1152/ajpregu.00782.2005. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Martin-Vasallo P, Sweadner KJ. Isoforms of Na, K-ATPase alpha and beta subunits in the rat cerebellum and in granule cell cultures. J Neurosci. 1997;17:3488–3502. doi: 10.1523/JNEUROSCI.17-10-03488.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips T, Rothstein JD. Glial cells in amyotrophic lateral sclerosis. Exp Neurol. 2014;262:111–120. doi: 10.1016/j.expneurol.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignataro G, Gala R, Cuomo O, Tortiglione A, Giaccio L, Castaldo P, Sirabella R, Matrone C, Canitano A, Amoroso S, Di Renzo G, Annunziato L. Two sodium/calcium exchanger gene products, NCX1 and NCX3, play a major role in the development of permanent focal cerebral ischemia. Stroke. 2004;35:2566–2570. doi: 10.1161/01.STR.0000143730.29964.93. [DOI] [PubMed] [Google Scholar]
- Pimentel VC, Zanini D, Cardoso AM, Schmatz R, Bagatini MD, Gutierres JM, Carvalho F, Gomes JL, Rubin M, Morsch VM, Moretto MB, Colino-Oliveira M, Sebastião AM, Schetinger MR. Hypoxia-ischemia alters nucleotide and nucleoside catabolism and Na+, K+-ATPase activity in the cerebral cortex of newborn rats. Neurochem Res. 2013;38:886–894. doi: 10.1007/s11064-013-0994-3. [DOI] [PubMed] [Google Scholar]
- Plotkin MD, Kaplan MR, Peterson LN, Gullans SR, Hebert SC, Delpire E. Expression of the Na+–K+–2Cl− cotransporter BSC2 in the nervous system. Am J Physiol. 1997a;272:C173–C183. doi: 10.1152/ajpcell.1997.272.1.C173. [DOI] [PubMed] [Google Scholar]
- Plotkin MD, Snyder EY, Hebert SC, Delpire E. Expression of the Na–K–2Cl cotransporter is developmentally regulated in postnatal rat brains: A possible mechanism underlying GABA’s excitatory role in immature brain. J Neurobiol. 1997b;33:781–795. doi: 10.1002/(sici)1097-4695(19971120)33:6<781::aid-neu6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Quednau BD, Nicoll DA, Philipson KD. Tissue specificity and alternative splicing of the Na+/Ca2+ exchanger isoforms NCX1, NCX2, and NCX3 in rat. Am J Physiol. 1997;272:C1250–C1261. doi: 10.1152/ajpcell.1997.272.4.C1250. [DOI] [PubMed] [Google Scholar]
- Reyes RC, Verkhratsky A, Parpura V. Plasmalemmal Na+/Ca2+ exchanger modulates Ca2+-dependent exocytotic release of glutamate from rat cortical astrocytes. Sodium dynamics: Another key to astroglial excitability? ASN Neuro. 2012:4. doi: 10.1042/AN20110059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB, Wu Y. Dynamic equilibrium of neurotransmitter transporters: Not just for reuptake anymore. J Neurophysiol. 2003;90:1363–1374. doi: 10.1152/jn.00317.2003. [DOI] [PubMed] [Google Scholar]
- Rivera C, Li H, Thomas-Crusells J, Lahtinen H, Viitanen T, Nanobashvili A, Kokaia Z, Airaksinen MS, Voipio J, Kaila K, Saarma M. BDNF-induced TrkB activation down-regulates the K+-Cl− cotransporter KCC2 and impairs neuronal Cl− extrusion. J Cell Biol. 2002;159:747–752. doi: 10.1083/jcb.200209011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodacker V, Toustrup-Jensen M, Vilsen B. Mutations Phe785Leu and Thr618Met in Na+, K+-ATPase, associated with familial rapid-onset dystonia parkinsonism, interfere with Na+ interaction by distinct mechanisms. J Biol Chem. 2006;281:18539–18548. doi: 10.1074/jbc.M601780200. [DOI] [PubMed] [Google Scholar]
- Rodríguez JJ, Butt AM, Gardenal E, Parpura V, Verkhratsky A. Complex and differential glial responses in Alzheimer’s disease and ageing. Curr Alzheimer Res. 2016;13:343–358. doi: 10.2174/1567205013666160229112911. [DOI] [PubMed] [Google Scholar]
- Rose CR, Chatton JY. Astrocyte sodium signaling and neuro-metabolic coupling in the brain. Neuroscience. 2016;26:121–134. doi: 10.1016/j.neuroscience.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Rose CR, Karus C. Two sides of the same coin: Sodium homeostasis and signaling in astrocytes under physiological and pathophysiological conditions. Glia. 2013;61:1191–1205. doi: 10.1002/glia.22492. [DOI] [PubMed] [Google Scholar]
- Rose CR, Ransom BR. Intracellular sodium homeostasis in rat hippocampal astrocytes. J Physiol. 1996;491:291–305. doi: 10.1113/jphysiol.1996.sp021216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Verkhratsky A. Principles of sodium homeostasis and sodium signalling in astroglia. Glia. 2016 doi: 10.1002/glia.22964. [DOI] [PubMed] [Google Scholar]
- Rose CR, Waxman SG, Ransom BR. Effects of glucose deprivation, chemical hypoxia, and simulated ischemia on Na+ homeostasis in rat spinal cord astrocytes. J Neuropsychiatr. 1998;18:3554–3562. doi: 10.1523/JNEUROSCI.18-10-03554.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux MJ, Supplisson S. Neuronal and glial glycine transporters have different stoichiometries. Neuron. 2000;25:373–383. doi: 10.1016/s0896-6273(00)80901-0. [DOI] [PubMed] [Google Scholar]
- Saito R, Kaneko E, Tanaka Y, Honda K, Matsuda T, Baba A, Komuro I, Kita S, Iwamoto T, Takano Y. Involvement of Na+/Ca2+ exchanger in pentylenetetrazol-induced convulsion by use of Na+/Ca2+ exchanger knockout mice. Biol Pharm Bull. 2009;32:1928–1930. doi: 10.1248/bpb.32.1928. [DOI] [PubMed] [Google Scholar]
- Scheiner-Bobis G. The sodium pump. Its molecular properties and mechanics of ion transport. Eur J Biochem. 2002;269:2424–2433. doi: 10.1046/j.1432-1033.2002.02909.x. [DOI] [PubMed] [Google Scholar]
- Scorziello A, Savoia C, Sisalli MJ, Adornetto A, Secondo A, Boscia F, Esposito A, Polishchuk EV, Polishchuk RS, Molinaro P, Carlucci A, Lignitto L, Di Renzo G, Feliciello A, Annunziato L. NCX3 regulates mitochondrial Ca(2+) handling through the AKAP121-anchored signaling complex and prevents hypoxia-induced neuronal death. J Cell Sci. 2013;126:5566–5577. doi: 10.1242/jcs.129668. [DOI] [PubMed] [Google Scholar]
- Secondo A, Pignataro G, Ambrosino P, Pannaccione A, Molinaro P, Boscia F, Cantile M, Cuomo O, Esposito A, Sisalli MJ, Scorziello A, Guida N, Anzilotti S, Fiorino F, Severino B, Santagada V, Caliendo G, Di Renzo G, Annunziato L. Pharmacological characterization of the newly synthesized 5-amino-N-butyl-2-(4-ethoxyphenoxy)-benzamide hydrochloride (BED) as a potent NCX3 inhibitor that worsens anoxic injury in cortical neurons, organotypic hippocampal cultures, and ischemic brain. ACS Chem Neurosci. 2015;6:1361–70. doi: 10.1021/acschemneuro.5b00043. [DOI] [PubMed] [Google Scholar]
- Secondo A, Staiano RI, Scorziello A, Sirabella R, Boscia F, Adornetto A, Valsecchi V, Molinaro P, Canzoniero LM, Di Renzo G, Annunziato L. BHK cells transfected with NCX3 are more resistant to hypoxia followed by reoxygenation than those transfected with NCX1 and NCX2: Possible relationship with mitochondrial membrane potential. Cell Calcium. 2007;42:521–35. doi: 10.1016/j.ceca.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Seifert G, Schilling K, Steinhäuser C. Astrocyte dysfunction in neurological disorders: A molecular perspective. Nat Rev Neurosci. 2006;7:194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- Shen HY, van Vliet EA, Bright KA, Hanthorn M, Lytle NK, Gorter J, Aronica E, Boison D. Glycine transporter 1 is a target for the treatment of epilepsy. Neuropharmacology. 2015;99:554–565. doi: 10.1016/j.neuropharm.2015.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Chanana V, Watters JJ, Ferrazzano P, Sun D. Role of sodium/hydrogen exchanger isoform 1 in microglial activation and proinflammatory responses in ischemic brains. J Neurochem. 2011;119:124–135. doi: 10.1111/j.1471-4159.2011.07403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirihai O, Smith P, Hammar K, Dagan D. Microglia generate external proton and potassium ion gradients utilizing a member of the H/K ATPase family. Glia. 1998;23:339–348. [PubMed] [Google Scholar]
- Siesjo BK. Cell damage in the brain: A speculative synthesis. J Cereb Blood Flow Metab. 1981;1:155–185. doi: 10.1038/jcbfm.1981.18. [DOI] [PubMed] [Google Scholar]
- Silver IA, Erecinska M. Energetic demands of the Na+/K+ ATPase in mammalian astrocytes. Glia. 1997;21:35–45. doi: 10.1002/(sici)1098-1136(199709)21:1<35::aid-glia4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Smith JP, Cunningham LA, Partridge LD. Coupling of AMPA receptors with the Na+/Ca2+ exchanger in cultured rat astrocytes. Brain Res. 2000;887:98–109. doi: 10.1016/s0006-8993(00)02973-5. [DOI] [PubMed] [Google Scholar]
- Sohn Y, Yoo KY, Park OK, Kwon SH, Lee CH, Choi JH, Hwang IK, Seo JY, Cho JH, Won MH. Na+/HCO3− cotransporter immunoreactivity changes in neurons and expresses in astrocytes in the gerbil hippocampal CA1 region after ischemia/reperfusion. Neurochem Res. 2011;36:2459–2469. doi: 10.1007/s11064-011-0572-5. [DOI] [PubMed] [Google Scholar]
- Soleimani M, Burnham CE. Na+:HCO(3−) cotransporters (NBC): Cloning and characterization. J Membr Biol. 2001;183:71–84. doi: 10.1007/s00232-001-0055-8. [DOI] [PubMed] [Google Scholar]
- Sokolow S, Luu SH, Headley AJ, Hanson AY, Kim T, Miller CA, Vinters HV, Gylys KH. High levels of synaptosomal Na(+)-Ca(2+) exchangers (NCX1, NCX2, NCX3) co-localized with amyloid-beta in human cerebral cortex affected by Alzheimer’s disease. Cell Calcium. 2011;49:208–16. doi: 10.1016/j.ceca.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub F, Stoffel M, Berger S, Eriskat J, Baethmann A. Treatment of vasogenic brain edema with the novel Cl− transport inhibitor torasemide. J Neurotrauma. 1994;11:679–690. doi: 10.1089/neu.1994.11.679. [DOI] [PubMed] [Google Scholar]
- Stys PK. White matter injury mechanisms. Curr Mol Med. 2004;4:113–130. doi: 10.2174/1566524043479220. [DOI] [PubMed] [Google Scholar]
- Su G, Kintner DB, Flagella M, Shull GE, Sun D. Astrocytes from Na(+)- K(+)-Cl(−) cotransporter-null mice exhibit absence of swelling and decrease in EAA release. Am J Physiol Cell Physiol. 2002b;282:C1147–C1160. doi: 10.1152/ajpcell.00538.2001. [DOI] [PubMed] [Google Scholar]
- Su G, Kintner DB, Sun D. Contribution of Na(+)-K(+)-Cl(−) cotransporter to high-[K(+)](o)- induced swelling and EAA release in astrocytes. Am J Physiol Cell Physiol. 2002a;282:C1136–C1146. doi: 10.1152/ajpcell.00478.2001. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Driscoll BF, Law MJ, Sokoloff L. Role of sodium and potassium ions in regulation of glucose metabolism in cultured astroglia. Proc Natl Acad Sci USA. 1995;92:4616–4620. doi: 10.1073/pnas.92.10.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekkok SB, Goldberg MP. Ampa/kainate receptor activation mediates hypoxic oligodendrocyte death and axonal injury in cerebral white matter. J Neurosci. 2001;21:4237–4248. doi: 10.1523/JNEUROSCI.21-12-04237.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga T, Ishizaki T, Narumiya S, Barber DL. p160ROCK mediates RhoA activation of Na-H exchange. EMBO J. 1998;17:4712–4722. doi: 10.1093/emboj/17.16.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong XP, Li XY, Zhou B, Shen W, Zhang ZJ, Xu TL, Duan S. Ca(2+) signaling evoked by activation of Na(+) channels and Na(+)/Ca(2+) exchangers is required for GABA-induced NG2 cell migration. J Cell Biol. 2009;186:113–128. doi: 10.1083/jcb.200811071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcelay E, Ibarreta D, Parrilla R, Ayuso MS, Martín-Requero A. Enhanced proliferation of lymphoblasts from patients with Alzheimer dementia associated with calmodulin-dependent activation of the Na+/H+ exchanger. Neurobiol Dis. 2001;8:289–298. doi: 10.1006/nbdi.2000.0381. [DOI] [PubMed] [Google Scholar]
- Verkhraski A. Glial physiology and pathophysiology. Wiley-Blackwell; 2013. p. 560. [Google Scholar]
- Verkhratsky A, Olabarria M, Noristani HN, Yeh CY, Rodriguez JJ. Astrocytes in Alzheimer’s disease. Neurotherapeutics. 2010;7:399–412. doi: 10.1016/j.nurt.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Parpura V, Pekna M, Pekny M, Sofroniew M. Glia in the pathogenesis of neurodegenerative diseases. Biochem Soc Trans. 2014;42:1291–1301. doi: 10.1042/BST20140107. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Rodríguez JJ, Parpura V. Astroglia in neurological diseases. Future Neurol. 2013;8:149–158. doi: 10.2217/fnl.12.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitvitsky VM, Garg SK, Keep RF, Albin RL, Banerjee R. Na+ and K+ ion imbalances in Alzheimer’s disease. Biochim Biophys Acta. 2012;1822:1671–1681. doi: 10.1016/j.bbadis.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ. Cerebral white matter injury of the premature infant—More common than you think. Pediatrics. 2003;112:176–180. doi: 10.1542/peds.112.1.176. [DOI] [PubMed] [Google Scholar]
- Vornov JJ, Thomas AG, Jo D. Protective effects of extracellular acidosis and blockage of sodium/hydrogen ion exchange during recovery from metabolic inhibition in neuronal tissue culture. J Neurochem. 1996;67:2379–2389. doi: 10.1046/j.1471-4159.1996.67062379.x. [DOI] [PubMed] [Google Scholar]
- Wake H, Watanabe M, Moorhouse AJ, Kanematsu T, Horibe S, Matsukawa N, Asai K, Ojika K, Hirata M, Nabekura J. Early changes in KCC2 phosphorylation in response to neuronal stress result in functional downregulation. J Neurosci. 2007;27:1642–1650. doi: 10.1523/JNEUROSCI.3104-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Liu Y, Zhou J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl Neurodegener. 2015;4:19. doi: 10.1186/s40035-015-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AG, Sanchez-Watts G, Emanuel JR, Levenson R. Cell-specific expression of mRNAs encoding Na+, K(+)-ATPase alpha- and beta-subunit isoforms within the rat central nervous system. Proc Natl Acad Sci USA. 1991;88:7425–7429. doi: 10.1073/pnas.88.16.7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke S, Thomas R, Allcock N, Fern R. Mechanism of acute ischemic injury of oligodendroglia in early myelinating white matter: The importance of astrocyte injury and glutamate release. J Neuropathol Exp Neurol. 2004;68:872–881. doi: 10.1093/jnen/63.8.872. [DOI] [PubMed] [Google Scholar]
- Willoughby JO, Mackenzie L, Broberg M, Thoren AE, Medvedev A, Sims NR, Nilsson M. Fluorocitrate-mediated astroglial dysfunction causes seizures. J Neurosci Res. 2003;74:160–166. doi: 10.1002/jnr.10743. [DOI] [PubMed] [Google Scholar]
- Wilson JX. Antioxidant defense of the brain: A role for astrocytes. Can J Physiol Pharmacol. 1997;75:1149–1163. [PubMed] [Google Scholar]
- Wu C, Sun D. GABA receptors in brain development, function, and injury. Metab Brain Dis. 2015;30:367–379. doi: 10.1007/s11011-014-9560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Dempsey RJ, Sun D. Na+–K+–Cl− cotransporter in rat focal cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:711–721. doi: 10.1097/00004647-200106000-00009. [DOI] [PubMed] [Google Scholar]
- Yan Y, Dempsey RJ, Sun D. Inhibition of Na+–K+–Cl− cotransporter during focal cerebral ischemia decreases edema and neuronal damage. Brain Res. 2003;961:22–31. doi: 10.1016/s0006-8993(02)03832-5. [DOI] [PubMed] [Google Scholar]
- Yao H, Gu XQ, Douglas RM, Haddad GG. Role of Na(+)/H(+) exchanger during O(2) deprivation in mouse CA1 neurons. Am J Physiol Cell Physiol. 2001;281:C1205–C1210. doi: 10.1152/ajpcell.2001.281.4.C1205. [DOI] [PubMed] [Google Scholar]
- Yao H, Ma E, Gu XQ, Haddad GG. Intracellular pH regulation of CA1 neurons in Na(+)/H(+) isoform 1 mutant mice. J Clin Invest. 1999;104:637–645. doi: 10.1172/JCI6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Chopp M, Zhang ZG. Oligodendrogenesis after cerebral ischemia. Front Cell Neurosci. 2013;7:201. doi: 10.3389/fncel.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Nepomuceno R, Gao X, Foley LM, Wang S, Begum G, Zhu W, Pigott VM, Falgoust LM, Kahle KT, Yang SS, Lin SH, Alper SL, Kevin Hitchens T, Hu S, Zhang Z, Sun D. Deletion of the WNK3-SPAK kinase complex in mice improves radiographic and clinical outcomes in malignant cerebral edema after ischemic stroke. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X16631561. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchero JB, Barres BA. Glia in mammalian development and disease. Development. 2015;142:3805–3809. doi: 10.1242/dev.129304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zundorf G, Reiser G. Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antiox Redox Signal. 2011;14:1275–1288. doi: 10.1089/ars.2010.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]