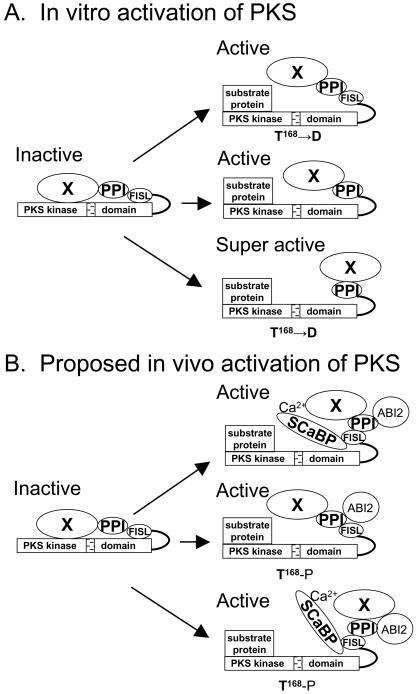
Figure 2.
Mechanisms of activation of PKS kinases in vitro and in vivo. PKS proteins consist of an N-terminal kinase catalytic domain (PKS kinase) and a C-terminal regulatory domain. An activation loop (stippled bar) is shown in the kinase catalytic domain, whereas the regulatory domain contains a FISL motif (for interaction with SCaBPs), a PPI motif (for interaction with protein phosphatase 2Cs), and an unknown functional motif(s) at the C-terminal end (indicated by X). The kinase is proposed to be kept inactive by an intramolecular interaction between the catalytic and regulatory domains. In vitro, the kinase can be activated by a T to D mutation in amino acid 168 to mimic phosphorylation of the activation loop by a putative upstream kinase (A, top panel), by deletion of the autoinhibitory FISL motif (A, middle panel), or by a combination of the two mutations (A, bottom panel). In vivo, the kinase is proposed to be activated by the binding of Ca2+-SCaBP to the FISL motif (B, to1p panel), by phosphorylation of T168 in the activation loop (B, middle panel), or by a combination of both (B, bottom panel).