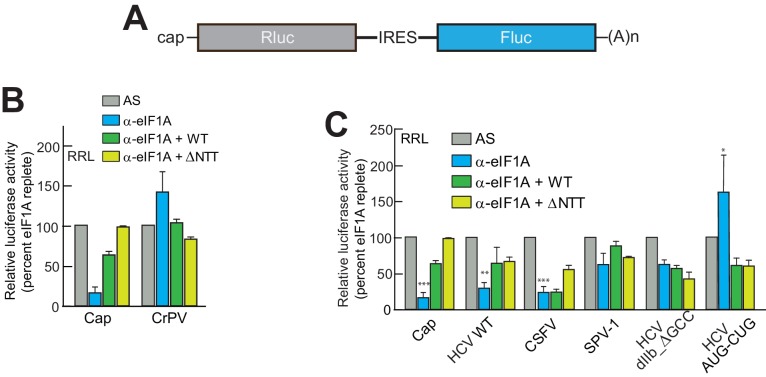
Figure 3. eIF1A depletion inhibits translation from the HCV IRES.
(A) Cartoon showing design of bicistronic dual-luciferase reporter constructs used in the experiments of panels (B–C). (B) Effect of depletion of eIF1A using the aptamer followed by add-back of WT full-length eIF1A (WT) or an N-terminally truncated mutant (ΔNTT) on translation from the reporter shown in panel (A). Experiments were done in RRL. This panel shows the effect on the Rluc (Cap) and a control CrPV IRES. (C) Same as panel (B) but with WT HCV, CSFV, and SPV-1 IRESs, plus two HCV IRES mutants shown in Figure 1B. For panels (B and C), activity in lysate treated with AS control aptamer was set at 100%. Error bars represent averages ±SEM of ≥3 independent experiments. Statistical significance shown by: *p<0.05, **p<0.01, ***p<0.001.