Abstract
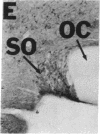
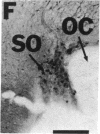
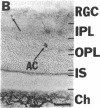
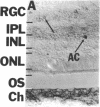
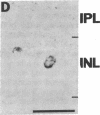
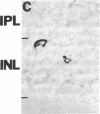
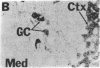
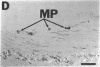
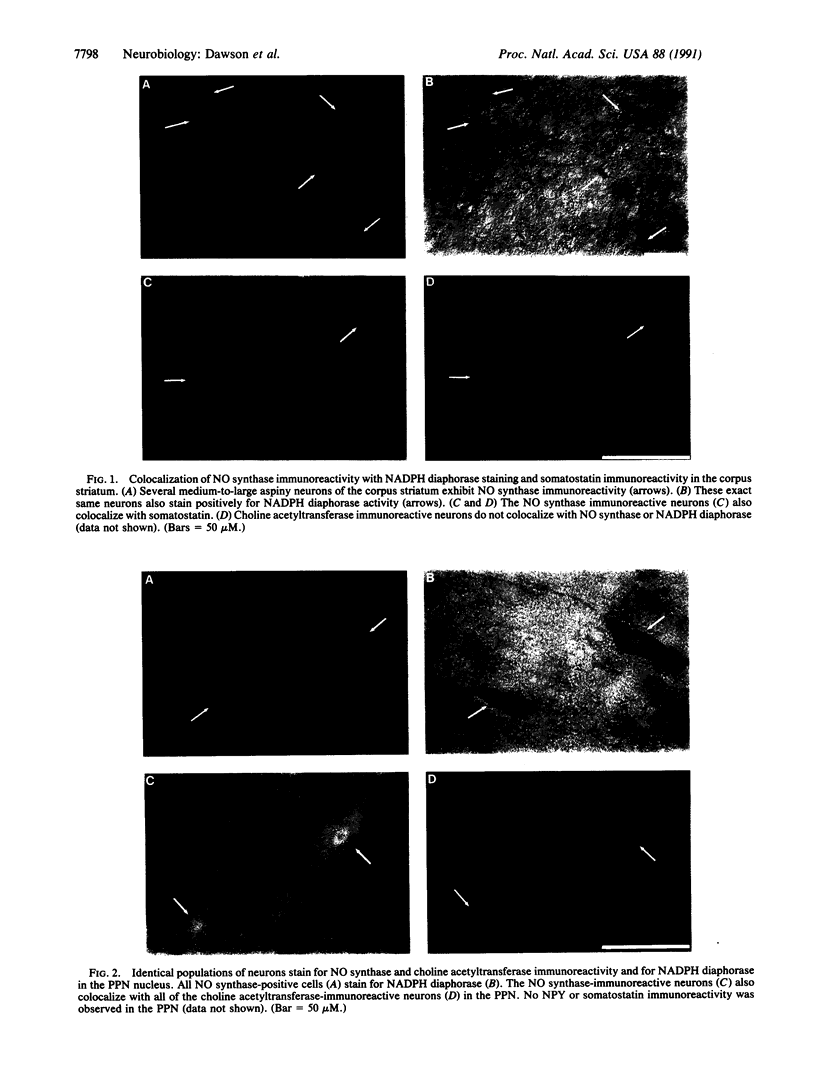
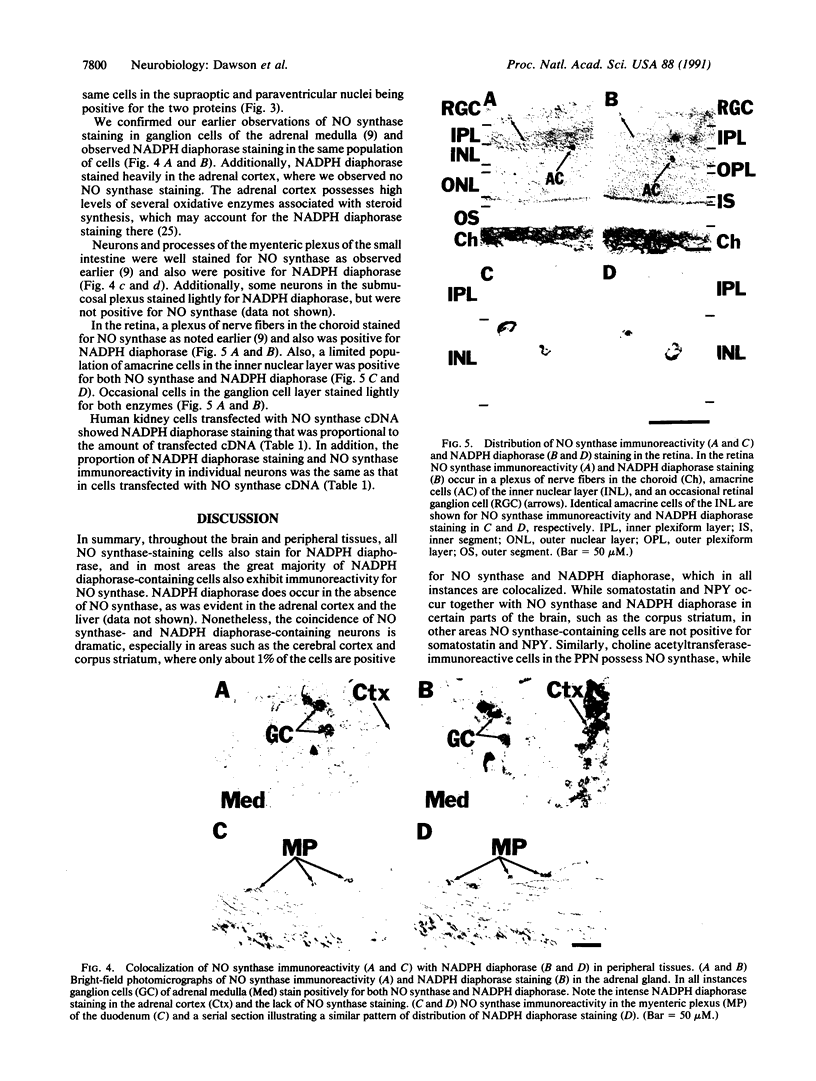
NADPH diaphorase staining neurons, uniquely resistant to toxic insults and neurodegenerative disorders, have been colocalized with neurons in the brain and peripheral tissue containing nitric oxide synthase (EC 1.14.23.-), which generates nitric oxide (NO), a recently identified neuronal messenger molecule. In the corpus striatum and cerebral cortex, NO synthase immunoreactivity and NADPH diaphorase staining are colocalized in medium to large aspiny neurons. These same neurons colocalize with somatostatin and neuropeptide Y immunoreactivity. NO synthase immunoreactivity and NADPH diaphorase staining are colocalized in the pedunculopontine nucleus with choline acetyltransferase-containing cells and are also colocalized in amacrine cells of the inner nuclear layer and ganglion cells of the retina, myenteric plexus neurons of the intestine, and ganglion cells of the adrenal medulla. Transfection of human kidney cells with NO synthase cDNA elicits NADPH diaphorase staining. The ratio of NO synthase to NADPH diaphorase staining in the transfected cells is the same as in neurons, indicating that NO synthase fully accounts for observed NADPH staining. The identity of neuronal NO synthase and NADPH diaphorase suggests a role for NO in modulating neurotoxicity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beal M. F., Kowall N. W., Ellison D. W., Mazurek M. F., Swartz K. J., Martin J. B. Replication of the neurochemical characteristics of Huntington's disease by quinolinic acid. Nature. 1986 May 8;321(6066):168–171. doi: 10.1038/321168a0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Snyder S. H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990 Oct 25;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson V. L., Dawson T. M., London E. D., Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante R. J., Kowall N. W., Beal M. F., Richardson E. P., Jr, Bird E. D., Martin J. B. Selective sparing of a class of striatal neurons in Huntington's disease. Science. 1985 Nov 1;230(4725):561–563. doi: 10.1126/science.2931802. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. The 1989 Ulf von Euler lecture. Studies on endothelium-dependent vasodilation and the endothelium-derived relaxing factor. Acta Physiol Scand. 1990 Jun;139(2):257–270. doi: 10.1111/j.1748-1716.1990.tb08923.x. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991 Feb;14(2):60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- Hope B. T., Michael G. J., Knigge K. M., Vincent S. R. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2811–2814. doi: 10.1073/pnas.88.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope B. T., Vincent S. R. Histochemical characterization of neuronal NADPH-diaphorase. J Histochem Cytochem. 1989 May;37(5):653–661. doi: 10.1177/37.5.2703701. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- Koh J. Y., Choi D. W. Vulnerability of cultured cortical neurons to damage by excitotoxins: differential susceptibility of neurons containing NADPH-diaphorase. J Neurosci. 1988 Jun;8(6):2153–2163. doi: 10.1523/JNEUROSCI.08-06-02153.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh J. Y., Peters S., Choi D. W. Neurons containing NADPH-diaphorase are selectively resistant to quinolinate toxicity. Science. 1986 Oct 3;234(4772):73–76. doi: 10.1126/science.2875522. [DOI] [PubMed] [Google Scholar]
- Kowall N. W., Beal M. F. Cortical somatostatin, neuropeptide Y, and NADPH diaphorase neurons: normal anatomy and alterations in Alzheimer's disease. Ann Neurol. 1988 Feb;23(2):105–114. doi: 10.1002/ana.410230202. [DOI] [PubMed] [Google Scholar]
- Kuonen D. R., Kemp M. C., Roberts P. J. Demonstration and biochemical characterisation of rat brain NADPH-dependent diaphorase. J Neurochem. 1988 Apr;50(4):1017–1025. doi: 10.1111/j.1471-4159.1988.tb10567.x. [DOI] [PubMed] [Google Scholar]
- Kwon N. S., Nathan C. F., Stuehr D. J. Reduced biopterin as a cofactor in the generation of nitrogen oxides by murine macrophages. J Biol Chem. 1989 Dec 5;264(34):20496–20501. [PubMed] [Google Scholar]
- Marletta M. A. Nitric oxide: biosynthesis and biological significance. Trends Biochem Sci. 1989 Dec;14(12):488–492. doi: 10.1016/0968-0004(89)90181-3. [DOI] [PubMed] [Google Scholar]
- Mizukawa K., Vincent S. R., McGeer P. L., McGeer E. G. Distribution of reduced-nicotinamide-adenine-dinucleotide-phosphate diaphorase-positive cells and fibers in the cat central nervous system. J Comp Neurol. 1989 Jan 8;279(2):281–311. doi: 10.1002/cne.902790210. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol. 1989 Jun 1;38(11):1709–1715. doi: 10.1016/0006-2952(89)90403-6. [DOI] [PubMed] [Google Scholar]
- Scherer-Singler U., Vincent S. R., Kimura H., McGeer E. G. Demonstration of a unique population of neurons with NADPH-diaphorase histochemistry. J Neurosci Methods. 1983 Nov;9(3):229–234. doi: 10.1016/0165-0270(83)90085-7. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Bredt D. S. Nitric oxide as a neuronal messenger. Trends Pharmacol Sci. 1991 Apr;12(4):125–128. doi: 10.1016/0165-6147(91)90526-x. [DOI] [PubMed] [Google Scholar]
- THOMAS E., PEARSE A. G. THE SOLITARY ACTIVE CELLS. HISTOCHEMICAL DEMONSTRATION OF DAMAGE-RESISTANT NERVE CELLS WITH A TPN-DIAPHORASE REACTION. Acta Neuropathol. 1964 Jan 2;3:238–249. doi: 10.1007/BF00684399. [DOI] [PubMed] [Google Scholar]
- THOMAS E., PEARSE A. G. The fine localization of dehydrogenases in the nervous system. Z Zellforch Microsk Anat Histochem. 1961;2:266–282. doi: 10.1007/BF00736504. [DOI] [PubMed] [Google Scholar]
- Tayeh M. A., Marletta M. A. Macrophage oxidation of L-arginine to nitric oxide, nitrite, and nitrate. Tetrahydrobiopterin is required as a cofactor. J Biol Chem. 1989 Nov 25;264(33):19654–19658. [PubMed] [Google Scholar]
- Uemura Y., Kowall N. W., Beal M. F. Selective sparing of NADPH-diaphorase-somatostatin-neuropeptide Y neurons in ischemic gerbil striatum. Ann Neurol. 1990 Jun;27(6):620–625. doi: 10.1002/ana.410270606. [DOI] [PubMed] [Google Scholar]
- Vincent S. R., Johansson O., Hökfelt T., Skirboll L., Elde R. P., Terenius L., Kimmel J., Goldstein M. NADPH-diaphorase: a selective histochemical marker for striatal neurons containing both somatostatin- and avian pancreatic polypeptide (APP)-like immunoreactivities. J Comp Neurol. 1983 Jul 1;217(3):252–263. doi: 10.1002/cne.902170303. [DOI] [PubMed] [Google Scholar]