Figure 4. CDK5 regulates cell invasion via its phosphorylation of KDR at Ser-229.
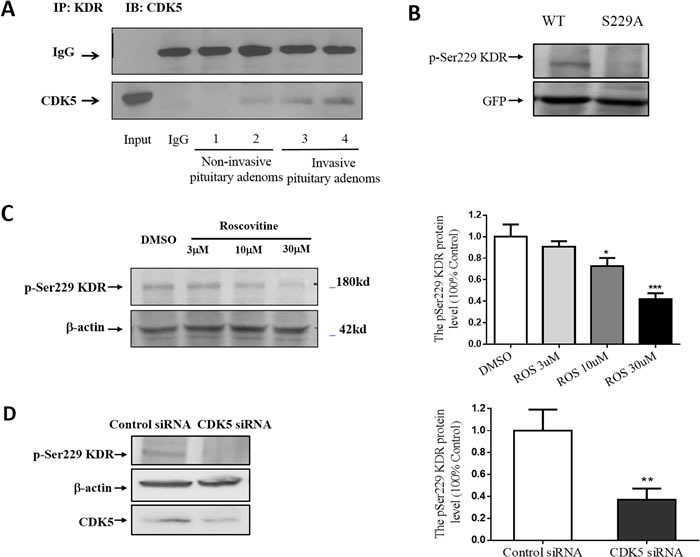
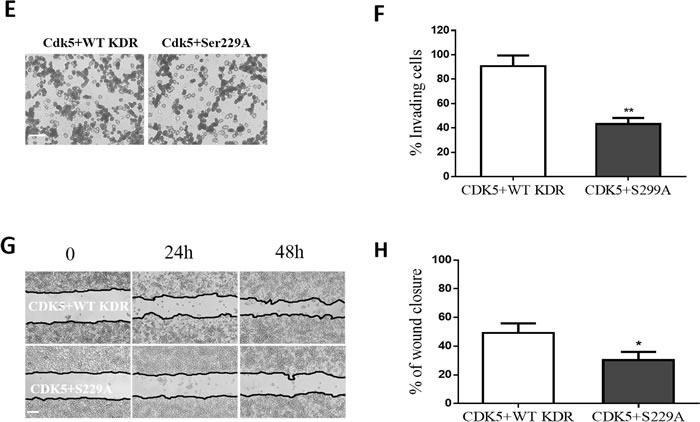
A. Interaction of CDK5 and KDR in prolactin pituitary adenomas. CDK5-specific antibody recognized the complex immunoprecipitated by KDR-specific antibody, but did not recognize normal IgG. B., GH3 pituitary cells were transfected with WT-KDR or S229A-KDR, and the blots were immunostained with site-specific pSer229 KDR antibody. Total cell lysates were immunoblotted with GFP and anti-actin as a loading control. C. Immunoblots (left) and densitometry analysis (right) showed KDR Ser-229 phosphorylation was reduced by roscovitine at indicated concentrations. β-actin served as a loading control. D. Immunoblots (left) and densitometry analysis (right) showed KDR Ser-229 phosphorylation was reduced by CDK5 siRNA at indicated concentrations. β-actin served as a loading control. E. Cell migration assay of GH3 pituitary cells cotransfected with CDK5 and WT-KDR/ S229A-KDR (200× magnification), scale bar, 20μm. F. Trend analysis of invasiveness in GH3 cells as indicated. G. Scratch wounds in monolayers of GH3 pituitary cell GH3 cells as indicated. Cells were observed by phase-contrast microscopy (100×), scale bar, 200μm. H: Cell migration into the scratch wound was quantified and presented as indicated. *P<0.05, **P<0.01, ***P<0.001 versus control cultures (two-tailed paired t test within each group. Error bars indicate SEM).
