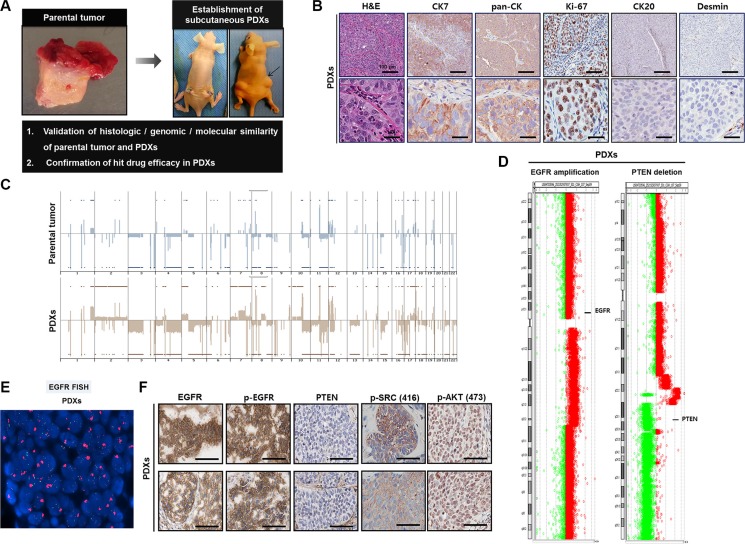
Figure 5. Establishment of patient-derived xenograft (PDX) models in 138T muscle-invasive bladder cancer (MIBC) and validation of the genetic, molecular, and histologic similarity between parental and PDX tumors.
(A) Schematic of the establishment of PDX tumors in 138T MIBC tissues. A PDX tumor was established by subcutaneous implantation in a BALB/c-nu mouse to validate the histologic, genomic, and molecular similarity of parental and PDX tumors. (B) Representative images of hematoxylin & eosin (H&E) and immunohistochemical (IHC) staining of 138T PDX tumor tissues with the indicated antibodies. CK7, cytokeratin 7; pan-CK, pan-cytokeratin; CK20, cytokeratin 20. Scale bars, 100 μm (Top), 20 μm (Bottom). (C) Array comparative genomic hybridization (CGH) analysis of 138T parental and PDX tumors. Gene amplifications and deletions were analyzed and compared between tumors to validate genetic similarity. (D) Array CGH analysis of 138T PDX tumors, illustrating the mutual EGFR amplification and PTEN deletion in the PDX and parental tumors. Individual chromosome ratio plots are shown with red representing the amplified region and green representing the deleted region. High-level EGFR amplification in chromosome 7 (left). PTEN deletion in chromosome 10 (right). (E) EGFR amplification assessed by FISH analysis in the 138T PDX tumor. Orange signal: EGFR; green signal: CEP7; blue signal: DAPI counterstaining. (F) Representative images of IHC staining of 138T PDX tumor tissue with EGFR, p-EGFR (Tyr1068), PTEN, p-SRC (Tyr416), and p-AKT (Ser473) antibodies. Scale bars, 100 μm.