Figure 5. Binding of VPS34 to TSC1 mediates TSC2 ubiquitination and degradation, and the activation of RheB.
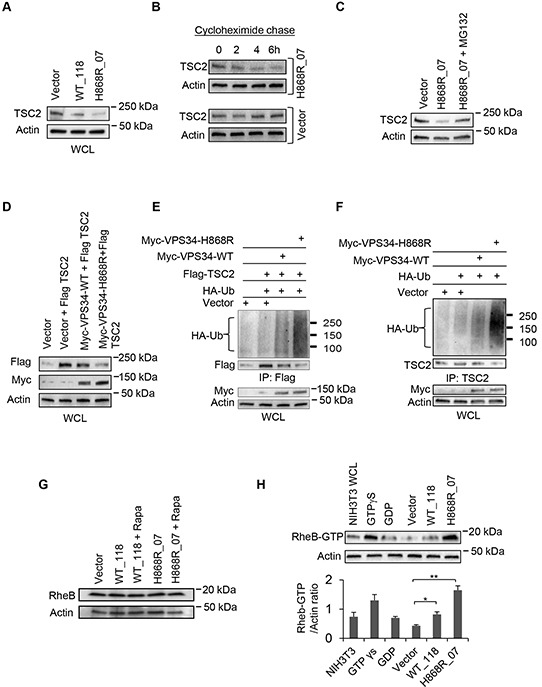
A. Western blot analysis was performed to detect the endogenous levels of TSC2 in Vector, WT_118 and H868R_07 stable lines using anti-TSC2 antibodies. B. Cycloheximide chase experiment was performed to monitor TSC2 degradation. Vector and H868R_07 stable cells were incubated with cycloheximide at 50 μg/ml for indicated times. After incubation, WCL was collected and western blotting was performed to detect the levels of TSC2 using anti-TSC2 antibody. C. Stably H868R_07 cells were treated with MG132 at 5 μM for 24h or left untreated. WCL was subjected to western blot analysis to observe the levels of TSC2 protein expression. D. COS7 cells were transiently co-transfected with plasmids encoding the indicated proteins. 48h post-transfection, Flag-tagged TSC2 and Myc-tagged proteins in WCL were detected by Western blotting using anti-Flag antibody and anti-Myc antibody, respectively. E. COS7 cells were transiently co-transfected with Vector or the indicated Myc-VPS34 constructs along with Flag-TSC2 and HA-Ubiquitin (HA-Ub). 48h post-transfection, Flag-tagged TSC2 was immunoprecipitated from WCL using an anti-Flag antibody. Ubiquitinated Flag-TSC2 was detected by anti-HA antibody and immunoprecipitated Flag-TSC2 was detected by anti-Flag antibody. Myc-tagged proteins in WCL were detected using anti-Myc antibody. F. NIH3T3 cells were transiently co-transfected with Vector or the indicated Myc-VPS34 constructs plus HA-Ubiquitin plasmid. 48h post-transfection, endogenous TSC2 was immunoprecipitated from WCL and ubiquitinated TSC2 was detected by Western blot using anti-HA antibody. The immunoprecipitated TSC2 was detected by Western blot using anti-TSC2 antibody for assessing TSC2 degradation. The protein levels of Myc-VPS34 proteins in the WCL were detected by Western blot using anti-Myc antibody. G. Expression of endogenous RheB was monitored in WCL of Vector, and WT_118 and H868R_07 cells, which were treated with rapamycin (10 nM) for 2 days or left untreated, by Western blot analysis using an anti-RheB antibody. H. RheB activation assay was performed to assess the levels of active RheB-GTP in Vector, WT_118 and H868R_07 cell clones. The RheB-GTP was detected by Western blot analysis using a rabbit polyclonal anti-RheB-GTP antibody. Quantitative analysis of RheB-GTP and actin was determined from three independent experiments and expressed as mean ± SEM (*, p < 0.05; **, p < 0.01).
