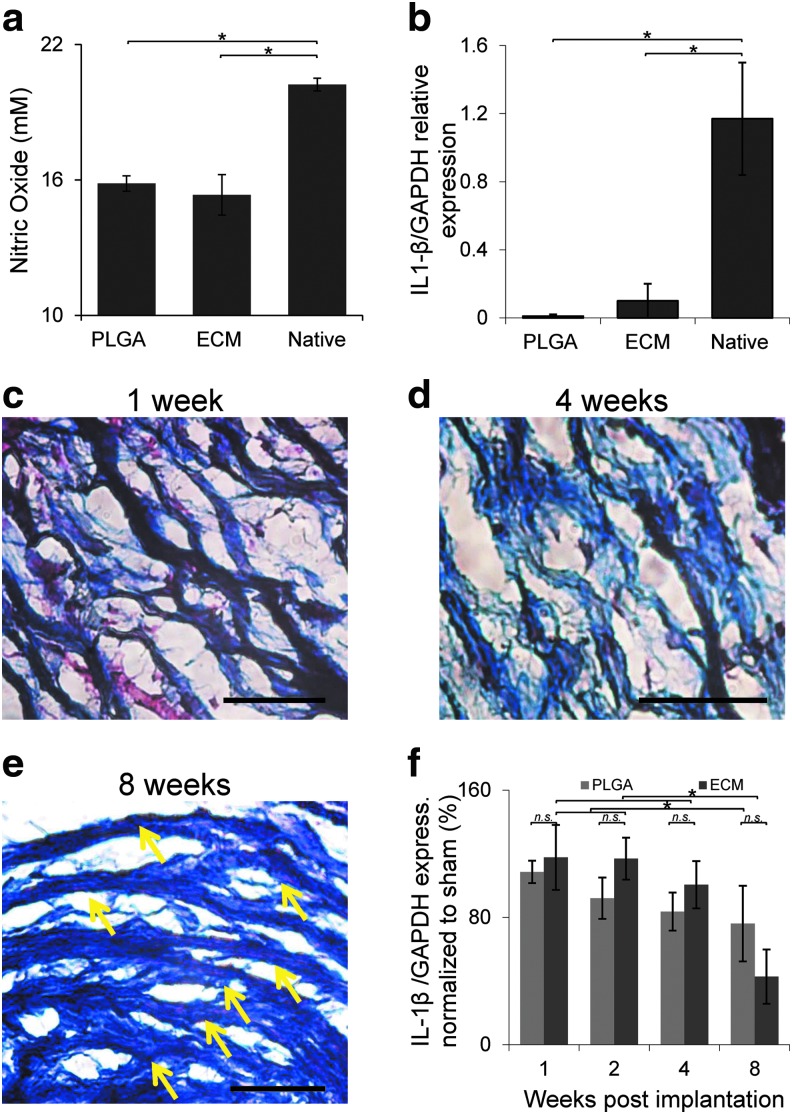
FIG. 2.
Assessment of decellularized implant immunoreactivity in vitro and biocompatibility in vivo. In vitro immunogenicity was evaluated by exposure of scaECM, FDA-approved PLGA, and native carotid tissue (control) to isolated primary MbmMΦs and quantifying their nitric oxide secretion (a) and expression of IL1B (a proinflammatory cytokine) normalized to housekeeping GAPDH (b). In vivo decellularized carotid arteries were subcutaneously implanted in C57 black mice. Representative MTC stains (red—cytoplasms; black—nuclei; blue—ECM fibers) of extracted implants (still largely intact) are shown at 1, 4, and 8 weeks postimplantation (c–e), respectively. No signs of encapsulation were apparent (Supplementary Fig. S2) and penetrating cells were well aligned to the scaECM fiber preferred direction at 8 weeks (indicated by yellow arrows). Xenogeneic ECM implants do elicit a similar to PLGA (negative control) inflammatory response whose kinetic profile is demonstrated by qPCR of IL1B-normalized expression in draining inguinal lymph nodes through time (f). *Denotes significant difference (p < 0.05), whereas n.s. denotes lack of statistical significance (p > 0.05); scale bars: 50 μm. In all assays, results represent a mean of n = 5 samples per group and images are representative of at least n = 3 images taken per sample. IL, interleukin; MbmMΦs, mouse bone marrow-derived macrophages; PLGA, poly-lactic-co-glycolic-acid; qPCR, quantitative polymerase chain reaction. Color images available online at www.liebertpub.com/tea