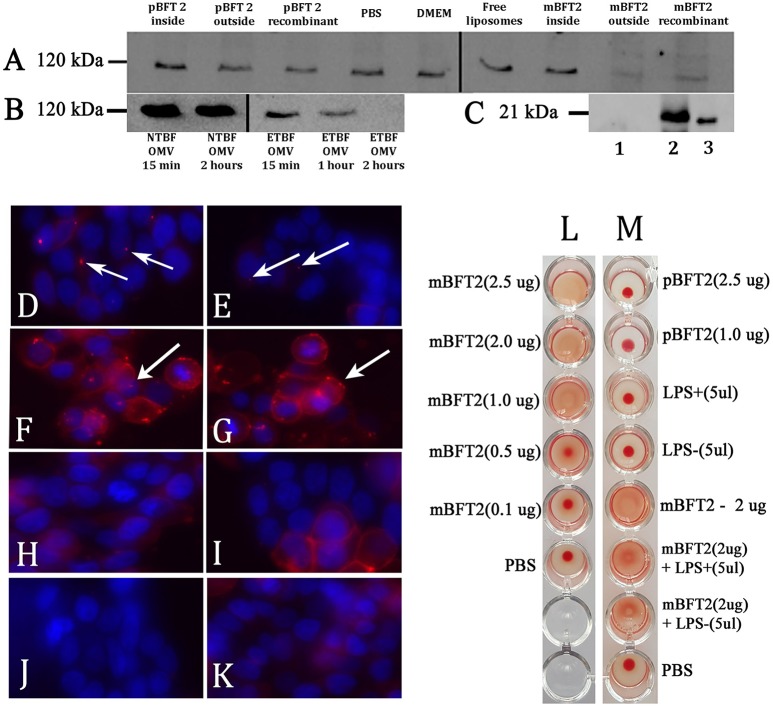
Figure 7.
Toxin containing liposomes and OMVs show proteolytic activity against HT-29 cell line E-cadherin and form complexes (A–K). Hemagglutination test (L–M) (A) Biological effects of the toxin to E-cadherin dependent of its localization. 12 nM pBFT2 and 14.2 nM mBFT2 encapsulated into liposome (pBFT2 inside and mBFT2 inside) or added to prepared liposomes—(pBFT2 outside and mBFT2 outside) were coincubated with HT-29 cells. Recombinant proteins were also coincubated with HT-29 cells (12 nM pBFT2–pBFT2 recombinant; 14.2 nM mBFT2–mBFT2 recombinant). PBS, DMEM and Free liposomes were used as negative controls. (B) Biological effect of the toxin containing OMVs to E-cadherin is time depended. 50 μg of total ETBF OMV proteins were coincubated with HT-29 cells for 15 min (ETBF OMV for 15 min), for 1 h (ETBF OMV for 1 h), for 2 h (ETBF OMV for 2 h). The same amounts of NTBF OMV proteins were coincubated with HT-29 cells for 15 min (NTBF OMV for 15 min) or for 2 h (NTBF OMV for 2 h). Extracted HT-29 cells proteins (A,B) were run on 10% SDS-PAGE followed by western blot with antibody against E-cadherin. (C) Toxin encapsulation and toxin-liposome association were examined by western blot. 14.2 nM mBFT2 with previously added prepared liposomes were dissolved in sterile culture media. After ultracentrifugation step supernatant was examined to unbound toxin—(lane 1), 14.2 nM mBFT2 encapsulated into the liposomes were treated with Proteinase K (20 ng/μl) and run on 10% SDS-PAGE followed by western blot with antibody against mBFT2–(lane 2). Recombinant protein—14.2 nM mBFT2—lane 3. (D–K) Complexes formation was examined by fluorescent microscopy. 3 μg OMVs, isolated from ETBF-(D) and NTBF-(E); 12 nM pBFT2-(F) and 14.2 nM mBFT2-(G) were added to 100 μl prepared liposomes; 14.2 nM mBFT2-(H) and 12 nM pBFT2-(I) were encapsulated into the liposomes. (J)-10 μl VybrantDiI were added to PBS and centrifuged at 100,000 g. (K)-100 μl liposomes. All samples (D–I, K) were labeled with VybrantDiI (red) and coincubated with HT-29 cells for 1 h. Nuclei were stained with DAPI (blue). Arrows indicate protein-lipid complexes located on cells surfaces. (L)-Hemagglutination activity of mBFT2 depends on toxin concentration. (M)-Hemagglutination activity of mBFT2 and pBFT2 in a presence of LPS.