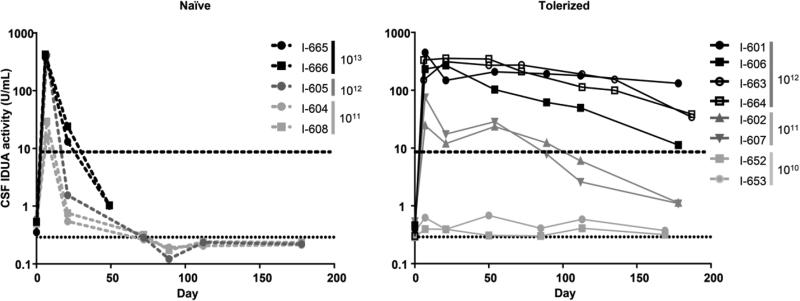
Figure 1.
CSF IDUA activity in MPS I dogs treated with intrathecal AAV9 expressing human IDUA. Dogs were treated at one month of age with an intrathecal injection of the vector into the cisterna magna. IDUA activity was measured in subsequent CSF samples. Vector doses (GC/kg) are indicated for each animal. The dashed lines represent animals treated with intrathecal vector only (left panel). The solid lines with filled symbols represent animals pretreated on postnatal day 5 with intravenous AAV8 expressing human IDUA from a liver-specific promoter (right panel, filled symbols). Solid lines with open symbols represent animals pretreated on postnatal day 7 and 14 with intravenous infusion of recombinant human IDUA (right panel, open symbols). Animals I-665 and I-666 were euthanized on day 36 due to neurological signs. The horizontal dashed line represents mean CSF IDUA activity in normal dogs. The horizontal dotted line indicates the assay limit of quantification.