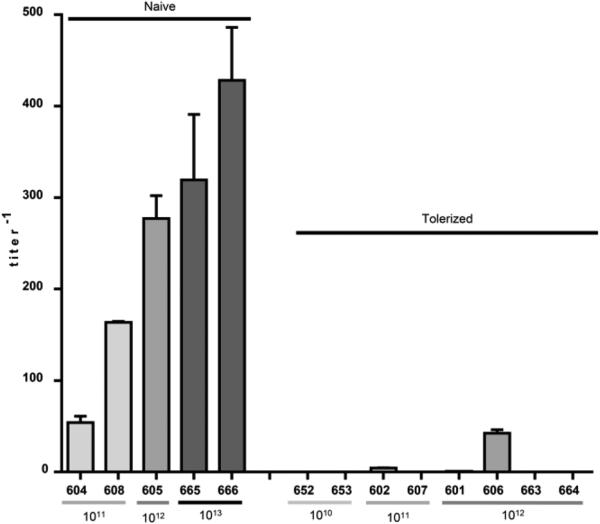
Figure 2.
CSF antibody titers against human IDUA. Antibody titers against human IDUA were measured by ELISA in CSF samples collected 50 days post vector administration. CSF samples tested from I-665 and I-666 were collected at the time of necropsy (day 36 post injection). Error bars = SEM. Antibody titers were significantly lower in the animals pre-treated as neonates with AAV8 vector (I-652, I-653, I-602, I-607, I-601, I-606) or recombinant human IDUA (I-663, I-664) compared with controls treated with IT vector alone (I-604, I-608, I-605, I-665, I-666) (p = 0.0016, two-tailed, Mann-Whitney test).