Abstract
Diabetes prevalence shows a continuous increasing trend in South Asia. Although well-established treatment modalities exist for type 2 diabetes mellitus (T2DM) management, they are limited by their side effect profile. Sodium–glucose co-transporter 2 inhibitors (SGLT2i) with their novel insulin-independent renal action provide improved glycemic control, supplemented by reduction in weight and blood pressure, and cardiovascular safety. Based on the clinical outcomes with SGLT2i in patients with T2DM, treatment strategies that make a “good clinical sense” are desirable. Considering the peculiar lifestyle, body types, dietary patterns (long duration religious fasts), and the hot climate of the South Asian population, a unanimous decision was taken to design specific, customized guidelines for T2DM treatment strategies in these regions. The panel met for a discussion three times so as to get a consensus for the guidelines, and only unanimous consensus was included. After careful consideration of the quality and strength of the available evidence, the executive summary of this consensus statement was developed based on the American Association of Clinical Endocrinologists/American College of Endocrinology protocol.
Keywords: Canagliflozin, dapagliflozin, diabetes mellitus, empagliflozin, glycosuria, hyperglycemia, sodium–glucose co-transporter 2, South Asia
EXECUTIVE SUMMARY: SODIUM–GLUCOSE CO-TRANSPORTER 2 INHIBITORS
A. BASIC PHARMACOLOGY
SGLT2i:
Increase urinary glucose excretion by lowering renal glucose reabsorption (Grade A, EL1)
Improve glycemic parameters (fasting, postprandial) with a low risk of hypoglycemia (the risk may increase with concomitant insulin/insulin secretagogues) (Grade A, EL1)
Reduce A1c with simultaneous reduction in weight and blood pressure (Grade A, EL1).
B. NOVEL MECHANISMS
SGLT2i:
Work as calorie restriction mimetics/nutrient offloaders (Grade C, EL4), thus reducing stress on β-cells and hyperinsulinemia (Grade B, EL2)
Promote adaptive ketogenesis (Grade B, EL2), providing a superfuel for tissues, including myocardium (Grade D, EL4)
Increase hematocrit and myocardial oxygen supply, without increasing the heart rate, thus improving cardiac efficiency (Grade D, EL4).
C. VASCULAR OUTCOMES
SGLT2i:
Reduce risk factors associated with macrovascular disease (Grade A, EL1)
Decrease death due to cardiovascular (CV) causes, all-cause mortality (Grade A, EL1), and hospitalization for heart failure (Grade B, EL1)*
Slow progression of nephropathy, thereby improving renal outcomes (Grade B, EL1)**.
D. CLINICAL PHARMACOLOGY
SGLT2i may be used as:
Dual or triple combination, in patients inadequately controlled on metformin or other antihyperglycemic agents (Grade A, EL1)
Combination therapy with insulin and may reduce insulin requirement***(Grade A, EL1).
Monotherapy in patients with contraindication to, or intolerance to, metformin (Grade B, EL1).
E. POSOLOGY
SGLT2i:
Are prescribed as once daily oral tablets (Grade A, EL1)
Require minimal dose titration (Grade A, EL1)
Have minimal drug interactions (Grade C, EL4).
F. PRAGMATIC USAGE
SGLT2i use:
Requires adequate fluid intake to prevent volume depletion. Cautious use recommended in frail elderly patients with concomitant use of diuretics (Grade A, EL1)
Requires maintenance of perineal hygiene to prevent genital tract infections (GTI). Avoid using in patients with history of recurrent GTI (>4/year) or history of recent upper urinary tract infection (UTI). During an episode of severe UTI, temporary discontinuation is recommended (Grade A, EL1)
Requires appropriate patient selection, medication counseling, and monitoring (Grade A, EL2).
G. CONTRAINDICATIONS
SGLT2i are contraindicated in:
Patients with an estimated glomerular filtration rate <45 mL/min/1.73 m2 (Grade A, EL1)
Patients with extreme insulinopenia or type-1 diabetes, on fluid/carbohydrate restricted diet, or with decompensated medical/surgical illness (Grade A, EL1)
Pregnancy, lactation, children (Grade A, EL1).
INTRODUCTION
Modern management of diabetes encompasses multiple metabolic goals and therapeutic targets, collectively aimed toward the prevention of complications of the disease.[1,2] Multiple risk factor intervention, targeting the control of glycemia, body weight, blood pressure (BP), and lipids, has convincingly demonstrated health benefits beyond the control of any single risk factor.[3] This, coupled with the multifactorial pathogenesis of diabetes, necessitates the use of either combination therapy or drugs with multi-dimensional modes of action that meet glycemic goals, manage microvascular outcomes, and attenuate cardiovascular risks. The World Health Organization (WHO) predicts the number of people with diabetes to go up from 171 million (2000) to 366 million (2030) worldwide.[4] In the South Asian region, the numbers are expected to be 119 million in 2030 from 46 million in 2011.[5]
Wide treatment options exist for diabetes and these are categorized into “clades,” which share a common mechanism of action. Thus, based on a cladistic approach, all noninsulin glucose-lowering drugs can be classified into three clades: insulin secretagogues, insulin sensitizers, and nutrient load reducers.[6] The third clade, nutrient load reducers, includes absorption inhibitors (α-glucosidase inhibitors [AGi], colesevelam, and orlistat) and excretion enhancer, sodium–glucose co-transporter 2 inhibitors (SGLT2i). The SGLT2i are the focus of this consensus document.
SODIUM–GLUCOSE CO-TRANSPORTER INHIBITORS
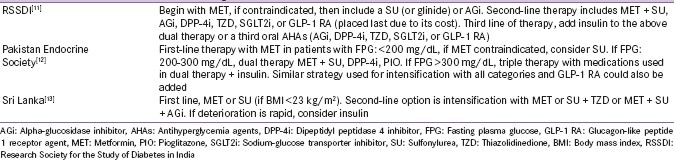
The SGLT2i, which prevent renal reabsorption of glucose, belong to the category of novel drugs that address metabolic pathways independent of impaired β-cell function and insulin resistance.[7,8] The European Medicines Agency (EMA) and Food and Drug Administration (FDA) have approved SGLT2i (canagliflozin, dapagliflozin, and empagliflozin) as monotherapy and as add-on to other antihyperglycemic agents (AHAs) [Table 1].[9] Ipragliflozin, luseogliflozin, and tofogliflozin have been approved in Japan for patients with type 2 diabetes mellitus (T2DM).[10] SGLT2i are available in India but not in Pakistan and Sri Lanka, and the T2DM management strategy of these South Asian countries is shown in Table S1.[11,12,13]
Table 1.
Overview of sodium-glucose co-transporter 2 inhibitors
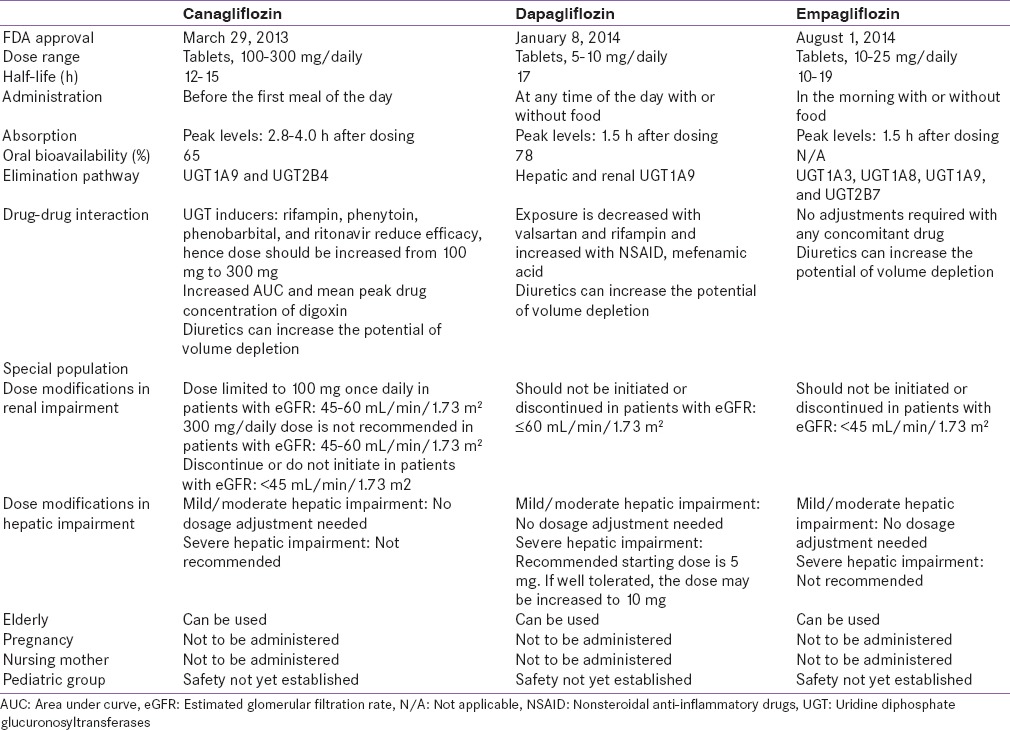
Table S1.
Recommendations for management of diabetes
SOUTH Asian Scenario
South Asia faces a high burden of T2DM, coupled with a unique metabolic phenotype and macro-environmental challenges. The prevalence of diabetes has risen most considerably in South Asia as compared to any other regions in the world, with diabetes occurring in people with lower body mass index (BMI) as compared to the western regions of the world.[14] As per the International Diabetes Federation Atlas (2015), in South-East Asian region, 78.3 million people live with diabetes (prevalence: 8.5%).[15] Increased life expectancy, increasing population growth, high rate of urbanization, low literacy, and inadequate national health expenditure are some reasons for the higher prevalence of diabetes in this region.[16]
South Asians have lower obesity rate (overweight: 14% in both sexes; obesity: 3%) based on the BMI; however, they tend to have a larger waist-to-hip ratio (central body obesity), which is associated with higher insulin levels and greater insulin resistance, thus predisposing them to T2DM and cardiovascular disease (CVD) risk as compared with Caucasians.[17,18] As per the WHO, CVD accounts for 3.7 million deaths (almost 25% of total deaths) in this region.[19] Hypertension is prevalent in 36% of South-East Asian adults and claims 1.5 million lives/year.[20] In South Asia, the metabolic syndrome (diabetes, hypertension, obesity, lipid disease [DHOL]) has increased in magnitude; akin to the loud beating of drums (DHOL: meaning drums in South Asia).
Due to limited resources, in developing countries of South Asia, self-monitoring of blood glucose (SMBG) is challenging. This should encourage the use of drugs with minimal risk of hypoglycemia. As the South Asian patient-to-doctor ratio is high, doctors have relatively less time for counseling. This leads them to refrain from prescribing drugs that need considerable titration of doses, monitoring, or follow-up. In general, the South Asian climate is hot and patients with T2DM are at a risk of dehydration in summer. Considering that SGLT2i can theoretically lead to volume depletion, control of dosing and monitoring when such drugs are prescribed is essential.
Long duration fasts (Ramadan, Jain Fasts, and Buddhist Lent) are observed by many people living in this region. Such fasts present a medical challenge as the risk of developing both hypoglycemia and dehydration increases. In view of these challenges, there is a need to have guidelines specifically addressing the South Asian population with regard to SGLT2i use in T2DM.
METHODOLOGY
The suggestions contained in the executive summary of this consensus statement were developed based on the American Association of Clinical Endocrinologists/American College of Endocrinology (AACE/ACE) protocol.[21] The panel met for a discussion three times (Kathmandu, Nepal July 23-24, 2016; Colombo, Sri Lanka August 11, 2016; and Kathmandu, Nepal September 9, 2016) so as to get a consensus for the guidelines, and only unanimous consensus was included.
An electronic literature search was conducted in the databases of MEDLINE and Cochrane Library. The search strategy was developed by combining Medical Subject Headings and free-text keywords using Boolean operators (“OR” and “AND”): sodium–glucose co-transporter type 2 inhibitors, canagliflozin, dapagliflozin, empagliflozin, type 2 diabetes mellitus. Articles from all clinical studies, reviews, systematic reviews, and meta-analysis were collected through October 2016, and no additional filters were used. The search was further intensified by performing a manual search of references from the relevant articles retrieved.
All the available literatures were discussed at the panel meeting and categorized as per the level of evidence based on the AACE, 2010 protocol (A-strong; B-intermediate; C-weak; and D-not evidence based) [Tables S2 and S3]. The guidelines are based on published evidence as well as experiential learning of the expert panel. The recommendations form a general guide to treating T2DM with SGLT2i in the South Asian population. While treating an individual patient, the benefits-risk potential must be analyzed and the line of therapy should be administered based on involved decision-making with the patient.
Table S2.
Evidence rating - 2010 American Association of Clinical Endocrinologists protocol for production of clinical practice guidelines

Table S3.
Recommendation grading - 2010 American Association of Clinical Endocrinologists protocol for the production of clinical practice guidelines

GLUCOSE HOMEOSTASIS
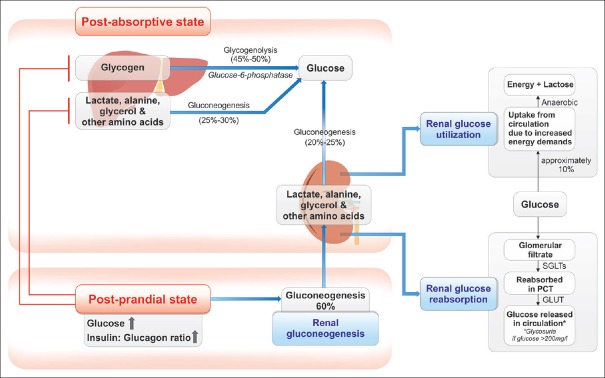
Glucose homeostasis in circulation through the kidneys is maintained by three processes [Figure 1].[22,23,24] In healthy individuals, the well-functioning kidneys filter approximately 180 g of glucose daily; all of this glucose is reabsorbed in the proximal convoluted tubule (PCT) of healthy individuals, thus leaving urine exempt of glucose. Kidneys contribute significantly to gluconeogenesis; however, the amount of glucose utilized by the kidneys for their own metabolism equates to a comparable proportion. Thus, the mechanism of renal glucose reabsorption exerts greater influence on blood glucose homeostasis, as compared to renal gluconeogenesis.[22]
Figure 1.
Glucose homeostasis. GLUTs: Glucose transporters, SGLTs: Sodium–glucose co-transporters
SODIUM–GLUCOSE Co-TRANSPORTERS
Receptor biology
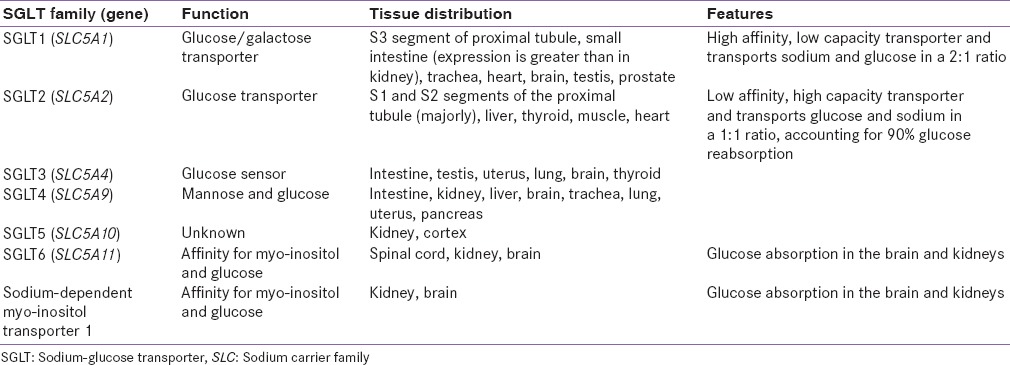
Renal glucose reabsorption is facilitated by SGLTs (6-member family) [Table 2]. The SGLTs, namely, SGLT1, SGLT2, SGLT4, SGLT5, SGLT6, and sodium-dependent myo-inositol transporter 1 are mainly expressed in the kidneys or intestine.[22,23,25,26,27]
Table 2.
Summary of sodium-glucose co-transporters
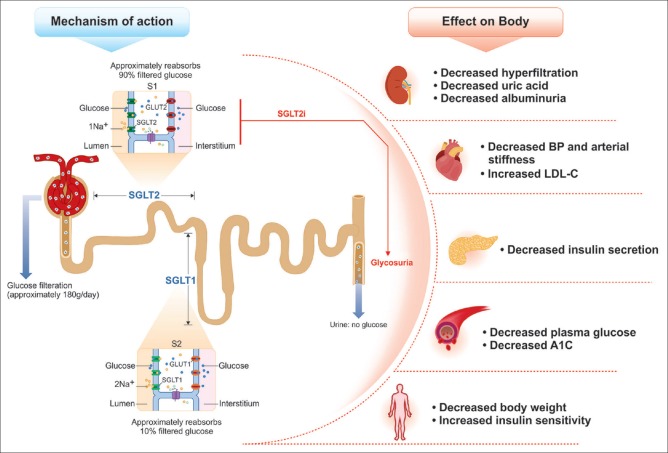
Glucose reabsorption in the kidneys occurs through the PCT; approximately 90% reabsorption is through SGLT2 and remaining 10% is through SGLT1 [Figure 2]. The glucose transported in the PCT by SGLT mediation is released into circulation by a passive transport across membranes through facilitative glucose transporters (GLUTs).[28] In diabetic patients, SGLT2 expression is upregulated leading to an increase in the renal threshold. In this case, urinary glucose reabsorption is increased despite the elevated plasma glucose levels and this leads to an increase in the hyperglycemic condition.[29]
Figure 2.
Mechanism of action and effects of sodium–glucose co-transporter 2 inhibitors on the body. BP: Blood pressure, GLUTs: Glucose transporters, LDL-C: Low-density lipoproteins, SGLTs: Sodium–glucose co-transporters
Basic pharmacology
In patients with T2DM, the SGLT2 transporter expression and activity is upregulated in the PCT. These transporters are inhibited by the SGLT2-inhibiting agents. This results in an immediate effect of lowering of hyperglycemia through reduced renal glucose reabsorption and greater urinary glucose excretion (UGE) [Figure 3a]. Following ongoing SGLT2i therapy, the persistent calorie loss results in metabolic adaptations that lead to weight loss, reduction in β-cell stress and hyperinsulinemia, improved insulin sensitivity, and insulin secretion rate. These mechanisms ensure control of the plasma glucose levels on a sustained basis, regardless of insulin resistance or β-cell dysfunction, leading to improvement of glycemic parameters.[30,31,32,33,34,35] As the plasma glucose concentrations decrease, the renal filtration of glucose reduces, and proportionately lesser amount of glucose is eliminated; hence the risk of hypoglycemia is very low with the SGLT2i agents.[36,37,38,39]
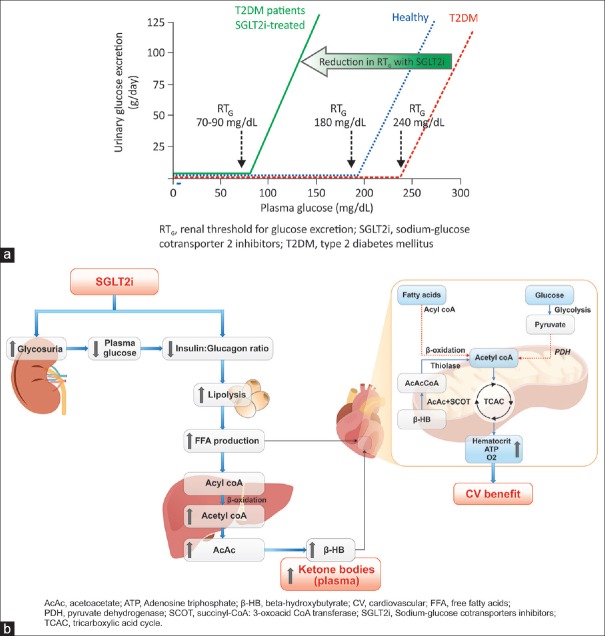
Figure 3.
Basic pharmacology of sodium–glucose co-transporter 2 inhibitors: (a) Renal threshold for glucose excretion and (b) ketogenesis. SGLT2i causing increased glycosuria and decreased IGR resulting in lipolysis and increased FFA concentration. Following hepatic uptake, FFA undergoes β-oxidation. The β-HB formed in liver is released in circulation and is then freely taken up by heart (in preference to FFA: dotted lines) and undergoes oxidative phosphorylation through TCAC. Excess acetyl-CoA restricts further formation of acetyl-CoA from pyruvate and from oxidation of fatty acids (dotted lines). This overall improves cardiac efficiency by releasing more oxygen and increasing hematocrit
Ketogenesis
In the recent cardiovascular outcome trial of empagliflozin, carried out in 7020 patients with T2DM and increased cardiovascular risk, a significant reduction in cardiovascular and overall mortality was evident with empagliflozin as compared with placebo, on the top of standard of care.[40] The contribution of various hypothesized mechanisms, toward this overall cardiovascular benefit, is currently under detailed assessment. One such hypothesized mechanism is the possible metabolic benefit, associated with mild-moderate levels of ketosis. Following SGLT2i treatment, the glycosuria results in reduced plasma glucose and insulin and some increase in glucagon levels. This condition resembles the fasting state, wherein a decrease in the insulin:glucagon ratio (IGR) causes the glucose-deprived body to shift to lipid utilization.[31,32] The SGLT2i thus have a ketogenic effect, which has been reported in both animal and human studies [Figure 3b].
Following the metabolic switch to fat metabolism, free-fatty acids (FFAs) are released in the circulation and mobilized to the liver, wherein they are converted into ketone bodies (acetoacetate, β-hydroxybutyrate, and acetone) in response to glucagon. These ketone bodies are hypothesized to contribute favorably to myocardial metabolism, particularly in the failing diabetic heart; ketone bodies are taken up by the heart, in preference to the FFA, and are oxidized through the tricarboxylic acid pathway in the mitochondria to generate adenosine triphosphate. The selection of ketone bodies as fuel improves the cardiac hydraulic efficiency (increased cardiac work with reduced oxygen consumption) of the diabetic or failing heart. Thus, ketone bodies generated by the SGLT2i therapy constitute a “thrifty substrate” that may be responsible for the beneficial cardiovascular outcomes.[41,42,43,44] Furthermore, as observed in this study, an increased hematocrit without a concomitant increase in the heart rate may result in improved oxygen supply to the myocardium without simultaneous increase in workload, thus contributing to myocardial efficiency.[45]
Dangers of ketogenesis in special situations
In cases of starvation, fasting, pregnancy, or gastrointestinal upset when the need for additional glucose arises, patients on SGLT2i therapy may fail to maintain homeostasis and ketosis may precipitate. A minimum of 100 g/daily carbohydrate intake has been observed to minimize ketogenesis in otherwise healthy situations. However, SGLT2i therapy increases the net calorie loss, hence adequate carbohydrate consumption is essential to minimize the risk of ketoacidosis.
Insulin:glucagon ratio
Insulin and glucagon act in contrasting manner to maintain homeostasis. Based on the metabolic status, individuals can be categorized into three types.[46,47] The healthy individuals, considered to be “eubolic,” with appropriate balance between catabolism and anabolism have a normal IGR. Persons with hyperinsulinemia (insulin resistance) have a high IGR and can be termed “maladaptively anabolic.” In such patients, overweight or obesity, high BP, dyslipidemia, and insulin resistance are observed. “Catabolic” patients have a low IGR with increased gluconeogenesis and glycogenolysis, exhibit asthenia, weight loss, cachexia, and malnutrition, and are characterized as insulin deficient. Using an IGR-based metabolic-fulcrum, a metabolic “triage” or classification of diabetes can be created to help inform treatment. A fall in IGR due to increase in glucagon and reduction in insulin levels is observed with SGLT2i treatment.[46,47] For maladaptive anabolism, therefore, SGLT2i are the preferred choice of treatment. However, SGLT2i can also be used in “eubolic” patients.
Calorie restriction mimetic action (nutrient offloading)
The primary mode of action of SGLT2i, i.e., calorie loss through urine, suggests a “calorie restriction mimetic” (CRM) action.[46] In tandem with this mechanism, the lowered IGR and pro-ketogenic effect of SGLT2i shift the focus from the strained carbohydrate utilization mechanism to the underused accumulated bounty of lipids.[31,32,46,48,49,50] This state may be considered a beneficial action that can rectify the “maladaptive anabolism” observed in obese T2DM patients.
Metformin, a well-known CRM, activates the carnitine palmitoyltransferase-1 pathway that facilitates the transport of FFAs in the mitochondria for β-oxidation, thereby leading to weight loss.[51] Apparently, both metformin and SGLT2i act in a similar fashion and aid in weight reduction by causing calorie loss. Following persistent SGLT2i therapy, some compensatory increase in calorie intake has been observed, resulting in plateauing of weight loss after 4-6 months. Stricter dietary control results in a greater weight loss with the SGLT2i agents. Although the weight reduction plateaus off over 4–6 months of therapy, the net reduction in weight is maintained, as long as the patient continues to receive the SGLT2i agent.[32]
Effect on glomerular filtration
Increased rates of glomerular filtration (125–140 mL/min/1.73 m2) and microalbuminuria have been regarded as prognostic markers of early renal dysregulation mediated by hyperglycemia and hypertension.[52,53] SGLT2i restore the tubuloglomerular feedback mechanism by lowering the sodium reabsorption in the proximal nephron leading to an increased supply of sodium to the distal juxtaglomerular apparatus. These effects are suggestive of renal protection as the lowering of intraglomerular pressure is a possible solution to a critical pathological pathway.[54,55,56]
In patients with type 1 diabetes mellitus, empagliflozin has demonstrated the ability to reduce hyperfiltration, activate tubuloglomerular feedback, and cause significant improvements in estimated glomerular filtration rate (eGFR).[54] In the EMPA-REG-OUTCOME study, which included patients with T2DM with high cardiovascular risk, a significantly lower risk for microvascular outcome events, progression of kidney disease (incident or worsening nephropathy), progression to macroalbuminuria, doubling of serum creatinine levels, and initiation of renal replacement therapy were observed in the empagliflozin-treated group versus placebo group.[56] Moreover, a significantly higher proportion of patients observed a regression of macroalbuminuria, as compared with placebo.[57]
Canagliflozin treatment has also achieved desired glycemic control without worsening renal function in Stage 3 chronic kidney disease (CKD) patients with T2DM.[58] Canagliflozin has demonstrated an improvement in urinary albumin-creatinine ratio and eGFR as compared with glimepiride over the period of 2 years, thus slowing the renal disease and providing reno-protective effects independent of its glycemic effect.[59] Dapagliflozin, on the other hand, has been studied only in patients with normal or mildly impaired renal functioning but it has been associated with favorable effects on renal end points.[60,61] In a retrospective meta-analysis, dapagliflozin has also demonstrated a reduction in proteinuria, suggestive of possible benefit for the proteinuria end point. Confirmatory evidence on the renal benefit of dapagliflozin is awaited from prospective trials.[60,61]
In the US vigilance database, 101 cases of acute kidney injury (AKI) and acute renal failure have been reported with canagliflozin and dapagliflozin; most cases improved after stopping the drugs.[62] Furthermore, in the EMPA-REG OUTCOME trial, in the first 30 days, slightly more events of AKI were reported in the empagliflozin-treated group (0.9%) versus the placebo group (0.7%); a similar trend was observed in the first 90 days (empagliflozin: 1.5% vs. placebo: 1.2%).[63] These signals highlight the importance of pragmatic use of SGLT2i to optimize the possible benefits and minimize any risk associated.
Intestinal sodium–glucose co-transporter 1 inhibition
In addition to SGLT2 inhibition, canagliflozin 300 mg transiently inhibits SGLT1, an important intestinal glucose transporter. Circulating concentrations of canagliflozin 300 mg are not expected to provide any meaningful systemic SGLT1 inhibition (based on the free [unbound] plasma concentrations of canagliflozin and the in vitro IC50).[64,65] However, during the periods of drug absorption, intestinal concentrations of canagliflozin 300 mg may be high enough to transiently inhibit intestinal SGLT1. In healthy individuals, canagliflozin 300 mg provides greater reductions in postprandial glucose (PPG) excursion and insulin that can be explained by the dual effect of increased UGE due to renal SGLT2 inhibition and delayed absorption of ingested glucose due to intestinal SGLT1 inhibition.[66,67] Similarly, in different Phase 3 trials where canagliflozin 300 mg was used as monotherapy/add-on to metformin/add-on to metformin + sulfonylureas (SU), similar reduction in PPG was reported, which validates the effect of canagliflozin on SGLT1. In a well-conducted Bayesian network meta-analysis (NMA) by Shyangdan et al., canagliflozin 300 mg showed the largest reduction in HbA1c (−1.23%) compared with placebo.[68] Compared with the other flozins, some differences appeared not only statistically significant but also clinically meaningful. However, how much of the added benefit with canagliflozin 300 mg is due to SGLT1 inhibition needs to be confirmed in larger clinical trials.
Novel metabolic mechanisms of SGLT2i

CLINICAL PHARMACOLOGY
Glycemic efficacy
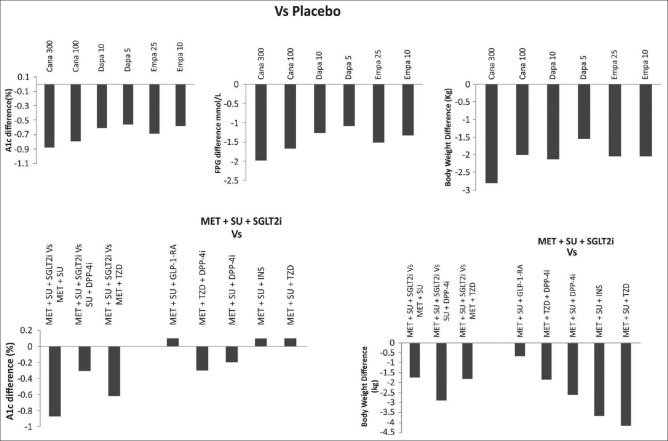
Clinical evidence supports the effective glucose- and A1c-lowering efficacy of SGLT2i [Figure 4].[68,69,70,71,72,73] In meta-analyses of randomized placebo-controlled studies, SGLT2i have demonstrated a significant reduction in A1c at weeks 12, 24, and 52 as compared with placebo, and comparable or superior A1c-reducing capacity versus active comparators at these time points.[69,70] Consistent efficacy and safety effects were demonstrated in short-term real-world setting and 1-year study conducted in the Indian population with T2DM receiving SGLT2i treatment.[74,75,76] Long-term durability of glycemic control, BP reduction, weight reduction, and maintenance of eGFR, combined with a sustained lower risk of hypoglycemia, have been demonstrated over 2 years of therapy with canagliflozin and 4 years of therapy with dapagliflozin and empagliflozin.[77,78,79]
Figure 4.
Efficacy of sodium–glucose co-transporter 2 inhibitors in clinical studies.[72,73] Cana: Canagliflozin, Dapa: Dapagliflozin, DPP-4i: Dipeptidyl peptidase 4 inhibitor, Empa: Empagliflozin, FPG: Fasting plasma glucose, GLP-1 RA: Glucagon-like peptide 1 receptor agent, INS: Insulin, MET: Metformin, SGLT2i: Sodium–glucose transporter inhibitor, SU: Sulfonylurea, TZD: Thiazolidinedione
Monotherapy
In treatment naïve patients, treatment with the SGLT2i agents as monotherapy, as adjuvants to diet and exercise, has demonstrated significant improvements in A1c.[78,80,81] In addition, in patients with higher baseline A1c (≥9% or 10%), significant and clinically relevant improvements in A1c levels have been reported following monotherapy with SGLT2i.[81,82,83]
Combination therapy with oral antihyperglycemic drugs
As add-on to other AHAs, SGLT2i agent supplementation leads to improved glycemic efficacy, body weight reduction, and BP lowering, with fewer events of hypoglycemia. In addition, in comparison with placebo, SGLT2i also improved cardiometabolic parameters.[73] In a NMA comparing the efficacy of SGLT2i versus liraglutide in patients (randomized control trials [RCTs] =17, n = 8784) with T2DM, inadequately controlled with metformin (alone or in combination with other oral AHAs), both doses of liraglutide showed statistically meaningful difference in A1c reductions with all comparators, except between liraglutide 1.2 mg and canagliflozin 300 mg.[84] All SGLT2i showed weight reductions in the same range as with liraglutide 1.8 mg, however weight reductions with liraglutide 1.2 mg were significantly lower than with canagliflozin 300 mg and empagliflozin 25 mg.
Dual therapy
In an NMA of SGLT2i therapy, all the currently available SGLT2i agents demonstrated consistent efficacy as an add-on to metformin, in terms of glycemia control, weight, and systolic blood pressure (SBP) reduction.[68] Canagliflozin has superiority over sitagliptin as greater proportion of patients receiving canagliflozin achieved composite target of A1c <7.5% and body weight reduction >3% (37.5%, 45%, and 19.2% proportion of patients achieving composite end point with canagliflozin 100 mg, canagliflozin 300 mg, and sitagliptin 100 mg, respectively).[85,86,87,88,89] Similar results were observed with canagliflozin as compared with glimepiride as add-on to metformin at 52 weeks (72.4% with canagliflozin 100 mg, 78.5% with canagliflozin 300 mg, and 26.8% with glimepiride).[90] Furthermore, in an NMA of dual therapy studies, A1c reductions were greater for canagliflozin 300 mg and similar for 100 mg in comparison with glucagon-like peptide 1 receptor agent (GLP-1 RA).[91] In an NMA of long-term studies (over 2 years) in patients (RCTs = 11) treated with canagliflozin as add-on to metformin, A1c reduction was greater for canagliflozin 300 mg compared with liraglutide 1.8 and 1.2 mg; weight reductions were similar between both the groups. In addition, glycemic efficacy was greater for canagliflozin 300 mg compared to dapagliflozin 10 and 5 mg and empagliflozin 25 mg.[89] In a study, dapagliflozin plus metformin therapy was noninferior and associated with reduction in body weight and fewer hypoglycemia versus glipizide plus metformin over 4 years.[77,92] In combination with metformin, empagliflozin (25 mg once daily) was compared to glimepiride as the second-line agent. In the initial 28 weeks of therapy, the HbA1c reduction was more pronounced with glimepiride; however, over the course of therapy, the glycemic control with empagliflozin was stable and greater as compared with glimepiride. Sustained improvements in body weight and BP at 104 weeks were observed with empagliflozin as compared to glimepiride.[93] Over 4 years of therapy, the superiority of empagliflozin relative to glimepiride, in terms of glycemic control, SBP, and weight reduction was maintained. In this 4-year extension study, empagliflozin was also associated with a better preservation of renal function, and much lower risk of hypoglycemia, relative to glimepiride.[79] In the absence of head-to-head trials comparing the efficacy aspects of the SGLT2i agents, a well-conducted Bayesian NMA by Shyangdan et al. suggested canagliflozin to be slightly better than other SGLT2i as monotherapy and similar efficacy with all the three available SGLT2i agents in dual therapy.[68]
Triple therapy
Comparison of canagliflozin as add-on therapy to metformin plus SU versus other AHAs (studies = 10; ~26 weeks duration) suggested that canagliflozin 300 mg was associated with increased A1c reduction versus dipeptidyl peptidase 4 inhibitor (DPP-4i) (sitagliptin and linagliptin) and was comparable to injectables, insulin, and GLP-1 RA (exenatide and liraglutide).[94] However, the efficacy of the 100 mg dose of canagliflozin was lower as compared to GLP-1 RAs and was comparable to the DPP-4i as a third-line therapy. As expected with the SGLT2i class, the weight reduction associated with both doses of canagliflozin was substantially higher than DPP-4i and insulin regimens and was comparable with GLP-1 RAs.[94] In triple-drug regimens, consistent effects have been noted with dapagliflozin (add-on to metformin and either sitagliptin/saxagliptin/SU) as well as with empagliflozin plus metformin and SU/pioglitazone (PIO).[95,96,97,98,99] As an add-on to metformin, the combination of empagliflozin (10 mg or 25 mg) and linagliptin (5 mg) has demonstrated consistently greater meaningful reductions in A1c over 52 weeks, as compared with the respective dual-therapy regimens. The reductions in body weight with these combinations were comparable to the empagliflozin–metformin dual-therapy regimens. Over 52 weeks, the observed incidence of hypoglycemia and urinary tract infection (UTI) events was not increased with the triple combination regimen.[100]
Combination therapy with insulin
Overall, as an add-on to oral AHAs or insulin, SGLT2i agents are of noteworthy relevance. These agents act on the components of fasting plasma glucose as well as PPG, resulting in lower dose requirements for the long-acting, as well as the short-acting insulins. They also reduce glucose load and resultantly insulin requirement. The risk of hypoglycemia is lower with these agents, and the insulin-related adverse effects of weight gain and fluid retention may be countered by these agents. These offerings of improved glycemic efficacy, coupled with lower risk of adverse effects, improve the therapeutic management of these patients.[101,102,103,104] However, these agents must not be considered as substitutes for insulin. Their use in severe insulinopenia, including type-1 diabetes, is associated with unfavorable insulin–glucagon balance and a higher risk of diabetic ketoacidosis (DKA). When initiating an SGLT2i agent, the dose of background insulin should be gradually down-titrated to lower the risk of hypoglycemia; a drastic reduction in the dose of insulin must not be attempted.
Adherence of canagliflozin-treated patients with T2DM was greater as compared with DPP-4i and GLP-1 RA when assessed in real-world scenario (The US claims data from Truven [66,206 patients] and Optum [19,536 patients] databases of insured patients); the increased persistence could be attributed to better effectiveness and/or tolerability.[105] As an add-on to insulin, canagliflozin in both the doses provided improved glycemic control with lowering of insulin requirement. Long-term studies of empagliflozin and dapagliflozin, in combination with various insulin regimens, have demonstrated lowering in insulin dose requirements by 10%–20%, as compared with placebo, with a good efficacy, safety, and tolerability profile.[101,102,103,104]
METABOLIC MODULATION
The unique β-cell independent mode of action of SGLT2i helps improve several cardiometabolic markers that may lead to favorable cardiovascular outcomes.
Effect on body weight
The current therapeutic options for the management of T2DM mainly target hyperglycemia alone, although evidence suggests that excess body weight is an equally important target of therapy. Some AHAs, particularly insulins, insulin secretagogues, and glitazones, are associated with considerable weight gain; thus incrementally diminishing their benefits on glycemia control.[106,107] This forms the basis of the “KgA1c paradox” with these agents, wherein Kg represents weight reduction and A1c levels are representative of glycemia. With the SGLT2i therapy, the calorie deficit and subsequent metabolic adaptations result in net weight loss, even in combination with insulin, SU, and PIO. The weight loss with SGLT2i therapy includes reductions in total body fat mass, visceral and subcutaneous adipose tissue.[40,108,109,110] Although metformin and incretin-based therapies do not demonstrate the KgA1c paradox, only the SGLT2i agents and GLP-1 RAs lead to an absolute reduction in body weight. More recently, a 21-year follow-up of the Steno-2 trial has demonstrated a sustained legacy effect of early multiple risk factor control, increasing the lifespan by nearly 8 years.[3] SGLT2i-based intervention classically targets the multiple cardiovascular risk factors in T2DM.
Effect on lipid profile
SGLT2i exhibit variable effects on the lipid profile. Observed effects include increase in high-density lipoprotein-cholesterol (HDL-C) and low-density lipoprotein-cholesterol (LDL-C) and reductions in triglycerides.[111,112] A meta-analysis of pooled data showed a significant increase in HDL-C levels with SGLT2i therapy and no clinically relevant changes in LDL-C and triglycerides.[69,70] A consistent increase in total cholesterol, LDL-C, and HDL-C levels has been demonstrated with all the SGLT2i agents. However, the ratio of LDL-C: HDL-C does not worsen.
Effect on uric acid
The uricosuric effect of SGLT2i may also contribute to the beneficial cardiovascular effects of SGLT2i, particularly for the renal disease. The mechanism of SGLT2 inhibition in the PCT results in an increased elimination of uric acid downstream. All the three SGLT2i agents have shown significant early reduction in uric acid levels.[40,113,114] However, their potential effect as uricosuric therapy has not yet been explored further.
HEMODYNAMIC MODULATION
Effect on blood pressure
Several pathways associated with the inherent action of SGLT2i have been postulated to improve BP. Osmotic diuresis, mild natriuresis, changes in nitric oxide release, and reductions in arterial stiffness contribute to lowering of BP.[115,116,117,118] Unlike the GLP-1 RAs, the SGLT2i do not trigger an increase in heart rate, despite reductions in BP.[40,119]
Pooled data from Phase 2 and 3 studies of SGLT2i have reported clinically meaningful reductions in SBP and diastolic BP.[68,115] Furthermore, it is encouraging to note that similar to glycemic control, body weight reductions and lowering of BP have been demonstrated over long treatment periods of up to 4 years.[77,79]
SAFETY
Genital and urinary tract infections
Genital tract infections (GTIs) are a common comorbid condition encountered in diabetes.[120] The glycosuria caused by SGLT2i predisposes them to the development of GTIs (balanitis and balanoposthitis in men and vulvovaginitis in women).[121,122,123,124] GTI occurs more commonly in uncircumcised men. Candida albicans was identified as the most common pathogen causing such infections. An increased incidence of GTIs with SGLT2i versus placebo (odds ratio [OR], 3.50 [confidence interval (CI), 2.46–4.99]) and active comparators (OR, 5.06 [CI, 3.44–7.45]) was reported; no differences were demonstrated among SGLT2i.[125] Post hoc analysis of data from Phase 3 studies of canagliflozin-treated patients with T2DM for the Indian subset demonstrated comparable incidence of GTIs in the Indian population vis-à-vis the overall population.[75] Consistent results have been observed in studies from other Asian countries.[126]
Although the risk of developing UTIs (urosepsis and pyelonephritis) was lesser than GTIs among SGLT2i-treated patients, UTIs in patients receiving SGLT2i were higher than those administered placebo (OR, 1.34 [CI, 1.03–1.74]).[125] A similar trend was observed for monotherapy with SGLT2i and in studies with SGLT2i and add-on combination therapy.[78,81,83,126] However, the risk of developing upper UTIs was minimal.[40,127] Both GTIs and UTIs were generally more common in females than males, mild to moderate in severity, rarely leading to treatment discontinuation and could be managed using standard treatment. Recurrent infections were rare in clinical studies, nevertheless caution needs to be exercised when recommending SGLT2i in patients with a history of recurrent UTIs or GTIs or those with anatomical obstruction to urinary tract.
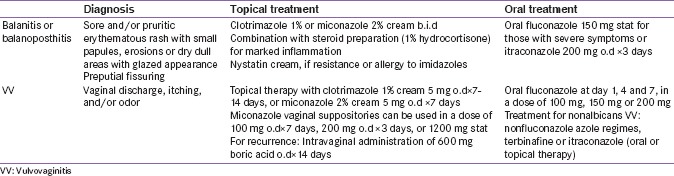
Considering the cultural background in South Asia, women would be hesitant to provide information regarding GTIs. Therefore, assurance of privacy and empathic obtaining of this information is essential. The diagnosis of vulvovaginitis is made through history, physical examination, and simple laboratory tests.[128,129] For the diagnosis of balanitis and balanoposthitis, a detailed history and physical examination is sufficient; biopsy is rarely necessary.[130] Local application of antifungal creams and powders can prove to be an effective treatment. Further, treatment strategies for GTIs are presented in Table S4, and the use of SGLT2i in patients with these infections is discussed in Appendix 1.[122,128,131,132]
Table S4.
Management of genital tract mycotic infections in patients treated with sodium-glucose transporter inhibitor
Volume depletion
Glycosuria resulting from SGLT2i therapy produces osmotic diuresis. Volume depletion-related AEs such as reduced BP, dehydration, postural dizziness, orthostatic hypotension, orthostatic intolerance, syncope, and reduced urine output were greater in patients treated with SGLT2i as compared with placebo or comparator, particularly in the frail elderly patients or those receiving strong diuretic agents concomitantly.[37,38,39] Assessment of volume status and thorough monitoring for hypotension and treatment adjustment is essential in these patients.
Hypoglycemia
By the virtue of their insulin-independent mechanism of action, SGLT2i have a low incidence of hypoglycemic episodes as compared with other AHAs. Monotherapy with SGLT2i or in combination with metformin or DPP-4i showed similar incidence of hypoglycemia risk as placebo. However, combination with insulin and SU may lead to hypoglycemia.[125] Thus, it is essential to monitor the incidence of hypoglycemia in patients on SGLT2i in combination with AHAs and suitable dose adjustments should be considered.
Euglycemic diabetic ketoacidosis
SGLT2i have ketogenic potential, and based on multiple case reports, drug safety communications were issued by the EMEA and FDA stating that SGLT2i may increase the risk of DKA.[133,134] However, off-label use in type 1 diabetes patients was considered a possible reason for this increased incidence of DKA in patients administered with SGLT2i. In some cases, precipitating events were also identified. An AACE/ACE position statement reported that the incidence of DKA in clinical trials of SGLT2i in patients with T2DM was low, and majority of the reported cases were in type 1 diabetes patients in whom SGLT2i use is not approved. Furthermore, the AACE/ACE emphasizes that the benefits of SGLT2i therapy outweigh the risks of DKA in T2DM patients and favor continued use of SGLT2i.[21] SGLT2i should be avoided in acute stressful situations, including an episode of illness, surgeries, or exhaustive exercise such as running a marathon. Maintenance of hydration, adequacy of carbohydrate consumption, avoidance of binge-drinking of alcohol, and maintenance of perineal hygiene are essential considerations to minimize the risk of DKA. The use should be avoided in severely insulinopenic patients, and if used concomitantly with insulin, the dose of insulin must be gradually down-titrated. A drastic reduction in insulin dose must be avoided.[21]
Ischemic events
While reviewing the ongoing CANVAS study, the EMEA noticed cases of lower limb amputation in both the canagliflozin and placebo groups.[135] However, when the results of the CANVAS study were compared with other completed canagliflozin studies (12 studies), no increase in such amputations was observed. The presence of other comorbidities in the CANVAS study amplifies this risk of toe amputations in patients with diabetes. Consequently, the EMEA and FDA had issued an alert regarding the same. Currently, the EMEA is reviewing the amputation risk with other SGLT2i class of drugs too and suggests that patients with diabetes with preexisting CVD might be at an increased risk of infection and ulceration, which can result in such ischemic events; however, the causal association between this signal and SGLT2i therapy is yet to be proved.[136]
Bone safety
Renal tubular mechanism of action of SGLT2i suggests the possibility of affecting bone mineral density (BMD) through alterations in calcium and phosphate homeostasis. SGLT2i have been observed to result in elevation in serum phosphate levels (mostly due to increased tubular resorption) and parathyroid hormone and simultaneously a decrease in the mean concentrations of 1,25-dihydroxyvitamin D.[137] Overall, this might result in impaired bone calcification, reduction in bone formation, and increase in bone resorption markers; consequently leading to increased risk of bone resorption markers.
A higher risk of bone fractures had been observed with dapagliflozin, particularly in patients with eGFR of <45 ml/min/1.73 m2 (CKD Stage 3b); however, dapagliflozin is not approved for use at this level of renal impairment.[138] Long-term studies in patients with the risk of osteoporosis such as those with renal insufficiency would provide additional evidence. A fracture imbalance was observed in patients treated with canagliflozin versus placebo within the first 26 weeks of therapy in CANVAS study (n = 4327 patients) conducted in patients with high risk for CVD. According to the EMEA, this observation was not consistent with the results from other T2DM studies with canagliflozin treatment (n = 5800 patients), as no adverse events (AEs) of BMD were noted even after 104 weeks of canagliflozin treatment.[139] Furthermore, the FDA suggested that the potential increase in fractures did not correlate (12–26 weeks) with the apparent decline in BMD, and the fractures limited to the upper extremity did not relate to bone fragility issue but rather may be because of falls.[37,38,39,140] A recent network and cumulative meta-analysis performed on data from RCTs (n = 30,384 patients) with 24–160-week follow-up noted that SGLT2i were not associated with an increased risk of fracture as compared with placebo, and the incidence of fracture events was similar for different SGLT2i.[141]
Bladder and breast cancer
Bladder cancer cases were observed with dapagliflozin therapy (four cases where study drug exposure was ≥ 1 year), which resulted in a warning in the prescribing information about contraindication in patients with active bladder cancer.[38] Similarly, a risk for breast cancer was identified in dapagliflozin-treated patients; however, these results could not be corroborated in clinical studies for canagliflozin and empagliflozin suggesting that it is not a class effect. It is noteworthy that the link between SGLT2i use and cancer risk is not conclusive, as molecular evidences and animal studies did not demonstrate a positive correlation between SGLT2i and cancer risk. It is postulated that cancer occurrence with dapagliflozin in humans could be due to early diagnosis of preexisting cancer.[142] The causal association between bladder cancer and dapagliflozin therapy is yet to be conclusively demonstrated; the results of ongoing DECLARE TIMI 58 trial will provide more definitive evidence.
COUNSELING
SGLT2i have been available for use for the past 3 years, and new efficacy and safety outcomes of this class of drugs are still emerging. Hence, it is important to educate the patients regarding (1) judicious use, (2) mechanism of action, (3) vigilance about the AEs, (4) identification of the symptoms for which immediate doctor's advice is essential, and (5) related first-aid measures [Table 3].
Table 3.
Counseling for risks associated with sodium-glucose co-transporter 2 inhibitor therapy

POSOLOGY
Considering the minimal risk of hypoglycemia, and once-daily dosing with sustained therapeutic coverage, SGLT2i have a positive influence on long-term patient adherence. Oral mode of administration with high bioavailability and permeability is another advantage.[143] Furthermore, additional benefits of weight loss, BP reduction, and uricosuria enable the usage of SGLT2i in patients with metabolic syndrome and mild fluid overload.
USE IN SPECIAL POPULATIONS
Elderly
Canagliflozin, dapagliflozin, and empagliflozin may be used cautiously in elderly patients with T2DM along with regular monitoring of volume status, BP, and renal function.[37,38,39] In clinical studies involving geriatric patients, SGLT2i have demonstrated efficacy comparable to results observed in younger patients. In the EMPA-REG OUTCOME study, the CV benefits observed with empagliflozin were consistent and favorable in the elderly age group.[40] However, risks of AEs related to volume depletion (hypotension, postural dizziness, orthostatic hypotension, syncope, and dehydration) urinary end point, GTIs, and UTIs were higher in the geriatric population (≥75 years of age) with compromised renal function and use of loop diuretics.[37,38,39,144,145,146] In addition, autonomic neuropathy could cause incomplete emptying of the urinary bladder and lead to increased risk of UTIs in elderly patients.
Pregnancy and lactation
Canagliflozin, dapagliflozin, and empagliflozin have been classified as pregnancy category C medications. These agents do not have adequate, well-controlled clinical studies supporting their use in pregnant or lactating women, hence should not be prescribed in these conditions.
Patients with renal impairment
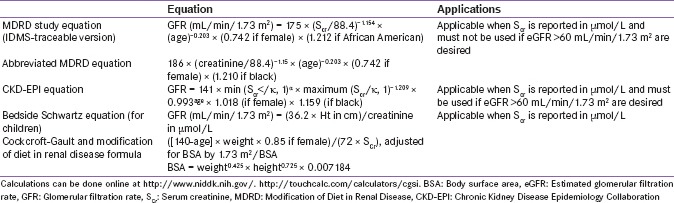
Treatment with canagliflozin, dapagliflozin, and empagliflozin is not recommended in patients with severe renal impairment (eGFR: <45 mL/min/1.73 m2), end-stage renal disease, or patients undergoing dialysis (Supplementary Table S5[147,148] shows calculation of GFR).[38,39,149]
Table S5.
Calculation of glomerular filtration rate
Intravascular volume depletion can be a cause for renal impairment, and the FDA has issued warning about the risk of developing AKI with canagliflozin and dapagliflozin treatment in patients with T2DM.[62] Factors such as reduced blood volume, chronic kidney insufficiency, and concomitant medications such as diuretics and BP medicines can predispose patients to AKI.[37,38,39] In such cases, the SGLT2i should be discontinued and treatment for AKI must be initiated. The observations from the EMPA-REG-OUTCOME study suggested a lower incidence rate of AKI with empagliflozin, as compared with placebo, regardless of the baseline kidney function status in patients with T2DM with high CV risk.[59] However, slightly higher risk of AKI with empagliflozin treatment as compared with placebo was observed in the early period of the study.[63] Further, the results from 2-year post hoc analysis of canagliflozin as an add-on to metformin have shown reassuring renal safety. These findings suggest that the long-term renal safety of SGLT2i therapy maybe reassuring and reinstate the importance of appropriate use of SGLT2i agents.
Patients with hepatic impairment
Canagliflozin, dapagliflozin, and empagliflozin were found to be stable in patients with mild-to-moderate hepatic impairment.[37,38,39] Hence, no dose adjustments are recommended in these populations. Treatment with canagliflozin and empagliflozin is not recommended in patients with severely impaired hepatic functioning. Further clinical data are needed to support the use of dapagliflozin in severely impaired hepatic functioning. Empagliflozin does not need dose adjustment in any stage of hepatic impairment.[37,38,39]
Patients with vascular disease
Diabetes is associated with greater CVD risk, and in patients with T2DM, the highest cause of mortality is CVD. However, the benefits of intensive glucose-lowering agents on CVD outcomes are variable.[150] In the EMPA-REG OUTCOME study, the primary composite outcome (death from CV causes, nonfatal myocardial infarction, or nonfatal stroke) occurred in 10.5% of patients in the pooled empagliflozin group versus 12.1% of patients in the placebo group (P = 0.04 for superiority).[40] Consistent with these results, significantly lower rates of death from CV causes (3.7%, vs. 5.9%) and hospitalization for heart failure (2.7% and 4.1%) were observed in the empagliflozin-treated group. There were no significant between-group differences in the rates of myocardial infarction or stroke.
In a meta-analysis of cardiovascular events performed on studies with canagliflozin treatment (Phase 2 = 1; Phase 3 = 8 studies including the CANVAS), an imbalance of major adverse cardiovascular events (MACE)-plus within the first 30 days of canagliflozin treatment was noted. As per the assessment of the US FDA, this could possibly be because of inappropriate patient selection, resulting in increased volume depletion-related events. Hence, a separate analysis of studies excluding the CANVAS study was conducted. No evidence of an increase in the risk of MACE-plus associated with canagliflozin treatment (n = 3510 patients) was observed in the non-CANVAS studies either in the first 30 days or overall (hazard ratio [HR]: 0.65 (0.35, 1.21).[140] Consistent results were observed in a meta-analysis of cardiovascular outcomes with dapagliflozin treatment (n = 5936 patients) (studies = 21 Phase 2b and Phase 3, including 2 studies with high CVD risk) for primary composite end point (HR: 0.81, 95% CI: 0.59–1.09) and MACE (HR: 0.78, 95% CI: 0.55–1.11).[151] Further support for the beneficial outcomes of reduced cardiovascular and all-cause mortality observed with empagliflozin extending to the class of SGLT2i (dapagliflozin, empagliflozin, canagliflozin, ipragliflozin, ertugliflozin, and luseogliflozin) was provided in a recent meta-analysis.[152] In addition, significant reduction in myocardial infarction with no increased risk of stroke was also observed in this study. The results of ongoing CV outcome trials (CANVAS, DECLARE TIMI 58) will shed more light on the CV benefit extending to the entire class of SGLT2i.
In South Asia, as the risk of CVD is high and availability of health resources is limited due to large population sizes, determination of relative risk of developing CVD in patients with T2DM is important. To delay or prevent complications, therapeutic measures can be systematically planned based on such risk stratification [Appendix 1 section includes details of QRISK and QDiabetes (heart failure) calculations].
Sodium–glucose co-transporter 2 inhibitors in hot climate
The South Asian region has hot climate for most of the year. Due to impairment of thermoregulatory mechanism and alterations in cutaneous vasodilation, orthostatic responses in patients with T2DM in such weather could be compromised.[153] Higher emergency department visits and hospitalizations are observed during hot weather in diabetes patients. It was thought that the increased osmotic diuresis, triggered by SGLT2i theoretically, may worsen volume depletion in summer.
Analysis of pooled data (randomized, double-blind, placebo-controlled, 26-week studies = 4; n = 611) from a subset of patients with T2DM residing in hot climate areas treated with canagliflozin (100 and 300 mg) demonstrated improved glycemic control and reduction in body weight and BP consistent with patients in the overall population.[154] No imbalance in the two populations was observed for volume depletion-related AEs. These outcomes suggest that SGLT2i are a safe option for patients with T2DM residing in hot climates including South Asia.
Fasting
Fasting due to religious reasons is a common practice in the South Asian region. General recommendations for Ramadan fasting include consultation with physician 3 months before fasting, regular monitoring of glucose during the fasting period, and reduction in physical activity during day time (when fasting).[155] Risks of hypoglycemia, hyperglycemia, DKA, and dehydration are well known during such fasting periods. In case hypoglycemia is observed, the fasting should be promptly discontinued. In a 12-week study conducted in patients with T2DM observing Ramadan fast, proportion of patients reporting hypoglycemia was significantly lower in the group of patients who switched to dapagliflozin plus metformin versus those who continued the SU plus metformin treatment (6.9% vs. 28.8%, P = 0.002).[156] In addition, no difference was observed for postural hypotension among the two groups. However, as these drugs cause fluid loss, precaution has to be exercised to prevent situations of dehydration.[157,158]
Hindu fasting periods have different durations ranging from half a day to 9–15 days. Prolonged Jain fasts not permitting food intake for 1–3 days and only boiled water during daytime are discouraged in persons taking SGLT2i. Patients taking SGLT2i may omit their therapy on the day of complete fasts. On the other hand, fasts that allow one or two meals during day time with boiled water or only abstinence from food and/or water after sunset can be practiced by the majority of healthy T2DM patients, provided appropriate regimen and dose adjustments are made. The evening dose can be a DPP-4i, SGLT2i, or an AGi. Change in the dosage and time of SGLT2i may not be required as they have a long half-life and low hyperglycemia risk. However, SGLT2i should be avoided during prolonged periods of abstinence from water. In case, gastrointestinal intolerance is observed, the administration time may be changed.[159]
PRAGMATIC USE
Based on the clinical outcomes with SGLT2i in patients with T2DM, treatment strategies that make a “good clinical sense” are desirable. There is a consensus that treatment approaches for patients with T2DM in South Asia should be “customized” considering the peculiar lifestyle, body types, and dietary patterns.
Initiation
For patients with recently diagnosed T2DM or with mild hyperglycemia (A1c <7.5%), metformin is recommended as the first-line therapy. However, in metformin-intolerant patients, SGLT2i is an acceptable alternative as initial monotherapy. Before initiation of SGLT2i treatment, it is imperative to assess the renal function (eGFR) of patients and it should be assessed periodically thereafter. In addition, the metabolic “triage” system discussed earlier can be used to choose the alternative therapy to metformin. Long-term glycemic treatment recommendations are shown in Figure 5.
Figure 5.
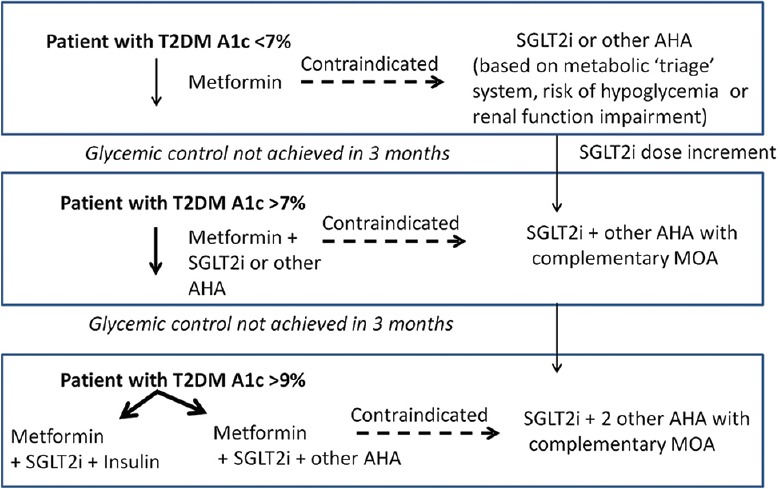
Pragmatic use of sodium–glucose co-transporter inhibitors. AHA: Anti-hyperglycemic agents, MOA: Mechanism of action, SGLT2i: Sodium–glucose co-transporter inhibitors, T2DM: Type-2 diabetes mellitus
Since the mode of action of SGLT2i in glucose lowering is independent of insulin secretion, they can be initiated at a later stage of the disease when insulin secretion capacity would have deteriorated. However, they should not be used as an alternative to insulin in severely insulinopenic patients.
Monitoring of therapy
Ideally, A1c should be measured for every 3 months, but not later than 6 months in patients with T2DM. For patients with T2DM receiving SGLT2i and who are not at target, SMBG should be performed in a structured manner (before meals and at bedtime) at least weekly to adjust effectiveness of therapy. Upon maintenance of the A1c target and absence of hypoglycemia, the SMBG can be performed less frequently.[160]
In patients undergoing insulin therapy, SMBG should be performed daily (minimum of twice daily and ideally before any insulin injection) and appropriate adjustments should be made to the insulin dosage. When adding noninsulin agents associated with elevated hypoglycemia risk (e.g., SU and glinides), SMBG should be performed at least weekly to confirm the effectiveness of therapy and detect possible hypoglycemia. The “start low, uptitrate slow” strategy is recommended. On achieving A1c target and absence of hypoglycemia events, SMBG can be performed less frequently.
Preference for sodium–glucose co-transporter 2 inhibitors
In addition to providing glycemic control, SGLT2i therapy may provide additional benefits in patients with T2DM and metabolic syndrome, in whom besides glycemic control, weight control or metabolic modulation and BP reduction is also desired [Table 4]. It can be prescribed to patients with eGFR >45 mL/min/1.73 m2, with no active GTI and UTI and those with a high risk of CVD. As the risk of hypoglycemia is rare with SGLT2i, the synergistic effect of SGLT2i along with other AHAs that have a propensity for hypoglycemia may be considered a better alternative.
Table 4.
Robust indication for sodium-glucose co-transporter 2 inhibitor

Canagliflozin should preferably be taken before the first meal of the day, once daily. Dapagliflozin and empagliflozin are taken once daily at any time of the day but at the same time daily.[37,38,39]
Interchange
If the first choice of AHAs (metformin, SU, or PIO) in dual therapy or triple therapy proves to be unsafe or poorly tolerated, they can be interchanged with SGLT2i based on the patients’ therapeutic requirement.
Intensification
If the glycemic goals are not met following 3 months of dual therapy, intensification can be achieved by adding SGLT2i to the preexisting therapy.[161]
PHARMACOVIGILANCE
As risks of DKA, UTIs/GTIs bone fractures, changes in BMD, and AKI with SGLT2i therapy were observed in clinical studies, long-term postmarketing surveillance studies to weigh the risks and benefits are essential.
CONCLUSIONS
Once daily, orally administered SGLT2i with their novel insulin-independent renal action provide glycemic control and additional benefits of reduction in weight and BP. The CV safety observed with SGLT2i agents supports the use of SGLT2i for combating multiple comorbid diseases associated with T2DM. Although GTI and to a lesser extent UTI were observed in few patients, they can be managed with appropriate counseling by the physician, suggesting that SGLT2i are generally well tolerated. The DKA risk observed in some patients was due to wrong patient selection which supports the pragmatic and safe use of SGLT2i in the management of T2DM, provided appropriate patient selection is ensured and adequate counseling is done.
Financial support and sponsorship
Funded by Janssen India (Pharmaceutical companies of Johnson & Johnson Pvt. Ltd.)
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We acknowledge Dr. Sangita P. Patil (CMPP™) for providing writing assistance and Dr. Madhavi Patil (both SIRO Clinpharm Pvt., Ltd.) for additional editorial support for the development of this manuscript. Dr. Ravi Santani and Dr. Amey Mane (Johnson & Johnson Pvt. Ltd., India) provided additional guidance and editorial support.
SUPPLEMENTARY APPENDIX 1
INITIATION
If there is a risk or previous history of occasional genital tract infection (GTI), sodium–glucose co-transporter 2 inhibitor (SGLT2i) can be prescribed if indicated. Maintenance of perennial hygiene and fluid balance should be advised. In cases of previous history of recurrent (>4/year) episodes of GTI and if SGLT2i is strongly indicated, in addition to perineal hygiene, prophylactic therapy, for example, fluconazole 150 mg/weekly [Table S4] should be considered. In contrast, if the patient has active GTI, SGLT2i should not be prescribed until infection is cleared. If asymptomatic bacteriuria occurs, SGLT2i can be prescribed (if strongly indicated) with concomitant antimicrobial therapy.
FOLLOW-UP OF THERAPY
Ongoing SGLT2i need not be discontinued if GTI occurs. The patient should be treated with appropriate antimicrobial therapy, and perineal hygiene must be reinforced. In cases of multiple episodes of GTI, prophylactic antifungal therapy along with perineal hygiene can be suggested. In addition, vigilance for other causes or risk factors predisposing to GTI is required. Recurrent episodes of GTI (>4/year), despite prophylactic antifungal therapy, should prompt discontinuation of SGLT2i. Similarly, single episode of upper urinary tract infection (UTI) would necessitate discontinuation of SGLT2i.
RECHALLENGE
Once the patient enjoys a GTI-free status for 3 months, SGLT2i can be prescribed along with prophylactic antifungal coverage. However, if the patient has a previous history of upper UTI, complicated GTI, or refractory or resistant GTI, avoid SGLT2i.
QRISK
Ethnicity-specific risk calculators have been developed in the United Kingdom for persons of Pakistani descent, living in the United Kingdom or in Pakistan. These QRISK web calculator helps calculate the risk of cardiovascular disease (CVD), over the next 10 years.[162] Only basic clinical information and demographic information is required, in addition to diabetes status, family history of cardiac or chronic kidney disease (CKD) (Stage 4 or 5) and rheumatoid arthritis. For example, if according to this calculator for a 50-year-old nonsmoking Pakistani male with treatment-10 year QRISK score: 25.2%; QRISK healthy heart age: 67; and relative risk: 3.6, this suggests that in the next 10 years, the chance of experiencing a myocardial infarction or stroke for such a person is 25.2%, which is 3.6 times the risk of a healthy 50-year-old's risk, and is equal to the risk of CVD of a healthy 67-year-old male Pakistani.[163] The markedly high risk, above the acceptable threshold, qualifies such a person for CVD treatment.
QDIABETES (HEART FAILURE)
The QDiabetes (heart failure) calculator requires information regarding age, sex, ethnicity, smoking status, whole years since the diagnosis of diabetes, type of diabetes (1 or 2), and A1c (mmol/mol).[164] If available, information about systolic BP, heart attack, angina or stoke, atrial fibrillation, CKD stage, cholesterol/high-density lipoprotein ratio, height and weight can be provided. Based on these inputs, it calculates the risk of a diabetes patient having heart failure within the next 10 years. For example, in a 50-year-old Bangladeshi nonsmoker female with type 2 diabetes mellitus for 1–3 years with A1c 70 mmol/mol, the risk for having heart failure in the next 10 years would be 5.3%. Thus, among 100 people with similar risk factors, 5 are likely to have heart failure in the next 10 years.
Footnotes
In the EMPA-REG-OUTCOME trial, empagliflozin demonstrated a reduction in death due to CV causes and all-cause mortality* (Grade A, EL1) and in hospitalizations for heart failure (Grade B, EL1). Other SGLT2i agents are likely to demonstrate these benefits, which would be confirmed in the ongoing trials (Grade B, EL4).
In the EMPA-REG-OUTCOME trial, empagliflozin demonstrated a reduction in the new onset or worsening of nephropathy, preservation of renal function, and regression of macroalbuminuria (Grade B, EL1). In the CANTATA SU trial, canagliflozin demonstrated an improvement in urinary albumin-creatinine ratio and eGFR; long-term preservation of renal function remains to be confirmed (Grade B, EL1). Dapagliflozin demonstrated a reduction in proteinuria in a retrospective analysis; evidence from prospective trials is awaited (Grade B, EL2).
If an SGLT2i is added to an insulin-based regimen, gradual down-titration of insulin dose is recommended.
REFERENCES
- 1.American Diabetes Association. Standards of medical care in diabetes-2016 abridged for primary care providers. Clin Diabetes. 2016;34:3–21. doi: 10.2337/diaclin.34.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm-2016 executive summary. Endocr Pract. 2016;22:84–113. doi: 10.4158/EP151126.CS. [DOI] [PubMed] [Google Scholar]
- 3.Gæde P, Oellgaard J, Carstensen B, Rossing P, Lund-Andersen H, Parving HH, et al. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow-up on the Steno-2 randomised trial. Diabetologia. 2016;59:2298–307. doi: 10.1007/s00125-016-4065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 5.Lam KS, Chow CC, Tan KC, Ma RC, Kong AP, Tong PC, et al. Practical considerations for the use of sodium-glucose co-transporter type 2 inhibitors in treating hyperglycemia in type 2 diabetes. Curr Med Res Opin. 2016;32:1097–108. doi: 10.1185/03007995.2016.1161608. [DOI] [PubMed] [Google Scholar]
- 6.Kalra S. Classification of non-insulin glucose lowering drugs. J Pak Med Assoc. 2016;66:1497–8. [PubMed] [Google Scholar]
- 7.McGill JB. Pharmacotherapy in type 2 diabetes: A functional schema for drug classification. Curr Diabetes Rev. 2012;8:257–67. doi: 10.2174/157339912800840541. [DOI] [PubMed] [Google Scholar]
- 8.Ukrainski M, Gandrabura T, Bischoff L, Ahmed I. On the horizon: New oral therapies for type 2 diabetes mellitus. Int J Diabetol Vasc Dis Res. 2013;1:15–8. [Google Scholar]
- 9.Nauck MA. Update on developments with SGLT2 inhibitors in the management of type 2 diabetes. Drug Des Devel Ther. 2014;8:1335–80. doi: 10.2147/DDDT.S50773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pharmaceuticals and Medical Devices Agency. Japan: 2013. [Last accessed on 2016 Aug 22]. Available from: http://www.pmda.go.jp/english/index.html . [Google Scholar]
- 11.Madhu SV, Saboo B, Makkar BM, Reddy GC, Jana J, Panda JK, et al. RSSDI Clinical Practice Recommendations for Management of Type 2 Diabetes Mellitus, 2015. Int J Diabetes Dev Ctries. 2015;35:1–71. [Google Scholar]
- 12.Guidelines for the management of type 2 diabetes mellitus in Pakistan. [Last accessed on 2016 Jun 29]. http://www.pakendosociety.org/dmguidelines/
- 13.Clinical Practice Guidelines: Management of Diabetes Mellitus. Sri Lanka: [Last accessed on 2016 Jun 29]. http://wwwslcoglk/img/guidelines/Other%20national%20Gidelines/Physicians/Book%201/Management%20of%20Diabetes%20Mellituspdf . [Google Scholar]
- 14.Ghaffar A, Reddy KS, Singhi M. Burden of non-communicable diseases in South Asia. BMJ. 2004;328:807–10. doi: 10.1136/bmj.328.7443.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.IDF Diabetes Atlas- South-Asian Region. [Last accessed on 2016 Jul 05]. Available from: http://www.diabetesatlas.org/resources/2015-atlas.html .
- 16.Jayawardena R, Ranasinghe P, Byrne NM, Soares MJ, Katulanda P, Hills AP. Prevalence and trends of the diabetes epidemic in South Asia: A systematic review and meta-analysis. BMC Public Health. 2012;12:380. doi: 10.1186/1471-2458-12-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unnikrishnan R, Anjana RM, Mohan V. Diabetes in South Asians: Is the phenotype different? Diabetes. 2014;63:53–5. doi: 10.2337/db13-1592. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Global Health Observatory (GHO) Data. 2008. [Last accessed on 2016 Jul 05]. Available from: http://www.who.int/gho/ncd/risk_factors/obesity_text/en/
- 19.World Health Organization Regional Office for South-East Asia. Cardiovascular Diseases Fact Sheet, Department of Noncommunicable Diseases and Environmental Health. [Last accessed on 2016 Sep 23]. Available from: http://www.searo.who.int/india/topics/cardiovascular_diseases/factsheet_cvd_2015.pdf .
- 20.World Health Organization Regional Office of South-East Asia. High Blood Pressure Global and Regional Overview. [Last accessed on 2016 Jul 05]. Available from: http://www.searo.who.int/entity/world_health_day/leaflet_burden_hbp_whd2013.pdf .
- 21.Handelsman Y, Henry RR, Bloomgarden ZT, Dagogo-Jack S, DeFronzo RA, Einhorn D, et al. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of SGLT-2 inhibitors and diabetic ketoacidosis. Endocr Pract. 2016;22:753–62. doi: 10.4158/EP161292.PS. [DOI] [PubMed] [Google Scholar]
- 22.Gerich JE. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: Therapeutic implications. Diabet Med. 2010;27:136–42. doi: 10.1111/j.1464-5491.2009.02894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med. 2007;261:32–43. doi: 10.1111/j.1365-2796.2006.01746.x. [DOI] [PubMed] [Google Scholar]
- 24.Farber SJ, Berger EY, Earle DP. Effect of diabetes and insulin of the maximum capacity of the renal tubules to reabsorb glucose. J Clin Invest. 1951;30:125–9. doi: 10.1172/JCI102424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright EM, Turk E. The sodium/glucose cotransport family SLC5. Pflugers Arch. 2004;447:510–8. doi: 10.1007/s00424-003-1063-6. [DOI] [PubMed] [Google Scholar]
- 26.Abdul-Ghani MA, DeFronzo RA, Norton L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30-50% of filtered glucose load in humans. Diabetes. 2013;62:3324–8. doi: 10.2337/db13-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown GK. Glucose transporters: Structure, function and consequences of deficiency. J Inherit Metab Dis. 2000;23:237–46. doi: 10.1023/a:1005632012591. [DOI] [PubMed] [Google Scholar]
- 28.Hediger MA, Rhoads DB. Molecular physiology of sodium-glucose cotransporters. Physiol Rev. 1994;74:993–1026. doi: 10.1152/physrev.1994.74.4.993. [DOI] [PubMed] [Google Scholar]
- 29.Sikaris K. The correlation of hemoglobin A1c to blood glucose. J Diabetes Sci Technol. 2009;3:429–38. doi: 10.1177/193229680900300305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bays H. Sodium glucose co-transporter type 2 (SGLT2) inhibitors: Targeting the kidney to improve glycemic control in diabetes mellitus. Diabetes Ther. 2013;4:195–220. doi: 10.1007/s13300-013-0042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124:499–508. doi: 10.1172/JCI72227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E. Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38:1730–5. doi: 10.2337/dc15-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalra S. Sodium glucose co-transporter-2 (SGLT2) inhibitors: A review of their basic and clinical pharmacology. Diabetes Ther. 2014;5:355–66. doi: 10.1007/s13300-014-0089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes. 2005;54:3427–34. doi: 10.2337/diabetes.54.12.3427. [DOI] [PubMed] [Google Scholar]
- 35.Sha S, Devineni D, Ghosh A, Polidori D, Chien S, Wexler D, et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab. 2011;13:669–72. doi: 10.1111/j.1463-1326.2011.01406.x. [DOI] [PubMed] [Google Scholar]
- 36.List JF, Whaley JM. Glucose dynamics and mechanistic implications of SGLT2 inhibitors in animals and humans. Kidney Int Suppl. 2011;120:S20–7. doi: 10.1038/ki.2010.512. [DOI] [PubMed] [Google Scholar]
- 37.Invokana Prescribing Information. 2016. May, [Last accessed on 2016 Jul 05]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/204042s015s019lbl.pdf .
- 38.Farxiga Prescribing Information. 2015. Dec, [Last accessed on 2016 Jul 05]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/202293s008lbl.pdf .
- 39.Jardiance Prescribing Information. 2016. Mar, [Last accessed on 2016 Jul 05]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/204629s005lbl.pdf .
- 40.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 41.Cahill GF., Jr Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258. [DOI] [PubMed] [Google Scholar]
- 42.Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: A “thrifty substrate” hypothesis. Diabetes Care. 2016;39:1108–14. doi: 10.2337/dc16-0330. [DOI] [PubMed] [Google Scholar]
- 43.McGarry JD, Foster DW. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- 44.Mudaliar S, Alloju S, Henry RR. Can a Shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study. A unifying hypothesis? Diabetes Care. 2016;39:1115–22. doi: 10.2337/dc16-0542. [DOI] [PubMed] [Google Scholar]
- 45.Sattar N, McLaren J, Kristensen SL, Preiss D, McMurray JJ. SGLT2 inhibition and cardiovascular events: Why did EMPA-REG outcomes surprise and what were the likely mechanisms? Diabetologia. 2016;59:1333–9. doi: 10.1007/s00125-016-3956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalra S, Jain A, Ved J, Unnikrishnan AG. Sodium-glucose cotransporter 2 inhibition and health benefits: The Robin Hood effect. Indian J Endocrinol Metab. 2016;20:725–9. doi: 10.4103/2230-8210.183826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalra S, Gupta Y. Choice of glucose-lowering therapy – A metabolic fulcrum-based approach. US Endocrinol. 2015;11:79–80. [Google Scholar]
- 48.Kalra S, Gupta Y, Patil S. Sodium-glucose cotransporter-2 inhibition and the insulin: Glucagon ratio: Unexplored dimensions. Indian J Endocrinol Metab. 2015;19:426–9. doi: 10.4103/2230-8210.152793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalra S, Sahay R, Gupta Y. Sodium glucose transporter 2 (SGLT2) inhibition and ketogenesis. Indian J Endocrinol Metab. 2015;19:524–8. doi: 10.4103/2230-8210.157859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124:509–14. doi: 10.1172/JCI70704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mora-Fernández C, Domínguez-Pimentel V, de Fuentes MM, Górriz JL, Martínez-Castelao A, Navarro-González JF. Diabetic kidney disease: From physiology to therapeutics. J Physiol. 2014;592:3997–4012. doi: 10.1113/jphysiol.2014.272328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maltese G, Abou-Saleh A, Gnudi L, Karalliedde J. Preventing diabetic renal disease: The potential reno-protective effects of SGLT2 inhibitors. Br J Diabetes Vasc Dis. 2015;15:114–8. [Google Scholar]
- 54.Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–97. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 55.Gnudi L, Karalliedde J. Beat it early: Putative renoprotective haemodynamic effects of oral hypoglycaemic agents. Nephrol Dial Transplant. 2016;31:1036–43. doi: 10.1093/ndt/gfv093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 57.Clinical Study Synopsis for Public Disclosure. [Last accessed in 2016 Sep 23]. Available from: http://www.trials.boehringer-ingelheim.com/content/dam/internet/opu/clinicaltrial/com_EN/results/1245/1245.25_c02695839-01_DR.pdf .
- 58.Yale JF, Bakris G, Cariou B, Yue D, David-Neto E, Xi L, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15:463–73. doi: 10.1111/dom.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heerspink HJ, Desai M, Jardine M, Balis D, Meininger G, Perkovic V. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol. 2016 doi: 10.1681/ASN.2016030278. pii: ASN.2016030278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kohan DE, Fioretto P, Johnsson K, Parikh S, Ptaszynska A, Ying L. The effect of dapagliflozin on renal function in patients with type 2 diabetes. J Nephrol. 2016;29:391–400. doi: 10.1007/s40620-016-0261-1. [DOI] [PubMed] [Google Scholar]
- 61.Heerspink HJ, Johnsson E, Gause-Nilsson I, Cain VA, Sjöström CD. Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin-angiotensin blockers. Diabetes Obes Metab. 2016;18:590–7. doi: 10.1111/dom.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.FDA Drug Safety Communication: FDA Strengthens Kidney Warnings for Diabetes Medicines Canagliflozin (Invokana, Invokamet) and Dapagliflozin (Farxiga, Xigduo XR) 2016. Jun, [Last accessed in 2016 Sep 23]. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm505860.htm .
- 63.FDA Briefing Document. (sNDA) 204629, Empagliflozin (JARDIANCE) Tablets, and sNDA 206111, Empagliflozin and Metformin Hydrochloride (SYNJARDY) Tablets. Boehringer Ingelheim Pharmaceuticals, and Metabolic Drug Advisory Inc., Endocrine Committee Meeting; 2016. Jun 28, [Last accessed in 2016 Oct 17]. Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM508422.pdf . [Google Scholar]
- 64.Devineni D, Curtin CR, Polidori D, Gutierrez MJ, Murphy J, Rusch S, et al. Pharmacokinetics and pharmacodynamics of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in subjects with type 2 diabetes mellitus. J Clin Pharmacol. 2013;53:601–10. doi: 10.1002/jcph.88. [DOI] [PubMed] [Google Scholar]
- 65.Liang Y, Arakawa K, Ueta K, Matsushita Y, Kuriyama C, Martin T, et al. Effect of canagliflozin on renal threshold for glucose, glycemia, and body weight in normal and diabetic animal models. PLoS One. 2012;7:e30555. doi: 10.1371/journal.pone.0030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Polidori D, Sha S, Mudaliar S, Ciaraldi TP, Ghosh A, Vaccaro N, et al. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: Results of a randomized, placebo-controlled study. Diabetes Care. 2013;36:2154–61. doi: 10.2337/dc12-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stein P, Berg JK, Morrow L, Polidori D, Artis E, Rusch S, et al. Canagliflozin, a sodium glucose co-transporter 2 inhibitor, reduces post-meal glucose excursion in patients with type 2 diabetes by a non-renal mechanism: Results of a randomized trial. Metabolism. 2014;63:1296–303. doi: 10.1016/j.metabol.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 68.Shyangdan DS, Uthman OA, Waugh N. SGLT-2 receptor inhibitors for treating patients with type 2 diabetes mellitus: A systematic review and network meta-analysis. BMJ Open. 2016;6:e009417. doi: 10.1136/bmjopen-2015-009417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berhan A, Barker A. Sodium glucose co-transport 2 inhibitors in the treatment of type 2 diabetes mellitus: A meta-analysis of randomized double-blind controlled trials. BMC Endocr Disord. 2013;13:58. doi: 10.1186/1472-6823-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: A meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2014;16:457–66. doi: 10.1111/dom.12244. [DOI] [PubMed] [Google Scholar]
- 71.Rydén L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, de Boer MJ, et al. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: Executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD) Eur Heart J. 2007;28:88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- 72.Downes MJ, Bettington EK, Gunton JE, Turkstra E. Triple therapy in type 2 diabetes; a systematic review and network meta-analysis. PeerJ. 2015;3:e1461. doi: 10.7717/peerj.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zaccardi F, Webb DR, Htike ZZ, Youssef D, Khunti K, Davies MJ. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes mellitus: Systematic review and network meta-analysis. Diabetes Obes Metab. 2016;18:783–94. doi: 10.1111/dom.12670. [DOI] [PubMed] [Google Scholar]
- 74.Baruah MP, Kalra S. Short-term outcomes of type 2 diabetes mellitus patients treated with canagliflozin in real-world setting. Indian J Endocrinol Metab. 2016;20:137–41. doi: 10.4103/2230-8210.172265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prasanna Kumar KM, Mohan V, Sethi B, Gandhi P, Bantwal G, Xie J, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus from India. Indian J Endocrinol Metab. 2016;20:372–80. doi: 10.4103/2230-8210.179996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sosale B, Sosale AR, Kumar PM, Joshi SR. A prospective analysis of the efficacy and safety of sodium glucose cotransporter 2 inhibitors: Real World Evidence from Clinical Practice in India. J Assoc Physicians India. 2016;64:40–4. [PubMed] [Google Scholar]
- 77.Del Prato S, Nauck M, Durán-Garcia S, Maffei L, Rohwedder K, Theuerkauf A, et al. Long-term glycaemic response and tolerability of dapagliflozin versus a sulphonylurea as add-on therapy to metformin in patients with type 2 diabetes: 4-year data. Diabetes Obes Metab. 2015;17:581–90. doi: 10.1111/dom.12459. [DOI] [PubMed] [Google Scholar]
- 78.Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: A randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33:2217–24. doi: 10.2337/dc10-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salsali A, Ridderstrale M, Toorawa R, Andersen KR, Woerle HJ. Empagliflozin Compared with Glimepiride as Add-on to Metformin for 4 Years in Patients with T2DM, Greater Red HbA1c and Hypoglycemia. American Diabetes Association 76th Scientific Sessions. New Orleans, Louisiana: 2016. Jun 10-14, [Google Scholar]
- 80.Stenlöf K, Cefalu WT, Kim KA, Jodar E, Alba M, Edwards R, et al. Long-term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes inadequately controlled with diet and exercise: Findings from the 52-week CANTATA-M study. Curr Med Res Opin. 2014;30:163–75. doi: 10.1185/03007995.2013.850066. [DOI] [PubMed] [Google Scholar]
- 81.Roden M, Weng J, Eilbracht J, Delafont B, Kim G, Woerle HJ, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1:208–19. doi: 10.1016/S2213-8587(13)70084-6. [DOI] [PubMed] [Google Scholar]
- 82.Skolnik N, Bonnes H, Yeh H, Katz A. Dapagliflozin in the treatment of patients with type 2 diabetes presenting with high baseline A1C. Postgrad Med. 2016;128:356–63. doi: 10.1080/00325481.2016.1173514. [DOI] [PubMed] [Google Scholar]
- 83.Stenlöf K, Cefalu WT, Kim KA, Alba M, Usiskin K, Tong C, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15:372–82. doi: 10.1111/dom.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lorenzi M, Jon Ploug U, Langer J, Skovgaard R, Jansen J. Liraglutide Versus SGLT2 Inhibitors in People with Type 2 Diabetes: A Network Meta-analysis. Poster – The World Diabetes Conference, 30 November-04 December. Vancouver, Canada: 2015. [Google Scholar]
- 85.Davidson JA, Schernthaner G, Hieronymus L, Jodon H, Vijapurkar U, Meininger G, et al. Canagliflozin is Superior to Sitagliptin in Reducing Both A1C and Body Weight in Patients with Type 2 Diabetes Mellitus. Poster – The 97th Annual Meeting and Expo of the Endocrine Society (ENDO) San Diego, California: 2015. Mar 05-08, [Google Scholar]
- 86.Langslet G, Davidson JA, Valentine V, Vijapurkar U, Canovatchel W, Meininger G. Canagliflozin reduces both HbA1c and body weight in patients with type 2 diabetes mellitus on background metformin. Poster – 50th Annual meeting of EASD. Austria: 2014. Sep, [Google Scholar]
- 87.Lavalle-González FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: A randomised trial. Diabetologia. 2013;56:2582–92. doi: 10.1007/s00125-013-3039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stein L, Schroeder M, Diels J, Hamilton G, Canovatchel W. London, UK: 2015. Mar 11-15, Greater Proportion of People with Type 2 Diabetes Inadequately Controlled on Metformin Reach the Composite Target of HbA1c<7.5% (59 mmol/mol) and >3% Body Weight Reduction when Treated with Either Canagliflozin 100 or 300 mg as Compared with Sitagliptin 100 mg at 52 weeks. Poster – The Diabetes UK Professional Conference. [Google Scholar]
- 89.Taieb V, Pacou M, Schroeder M, Nielsen AT, Schubert A, Neslusan C. A Network Meta-analysis to Assess the Longer-term Relative Efficacy of Canagliflozin in Patients with Type 2 Diabetes Inadequately Controlled on Metformin. Poster – The International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 18th Annual European Congress. Milan, Italy: 2015. Nov 07-11, [Google Scholar]
- 90.Leiter LA, Langslet G, Vijapurkar U, Davies MJ, Canovatchel W. Simultaneous reduction in both HbA1c and body weight with canagliflozin versus glimepiride in patients with type 2 diabetes on metformin. Diabetes Ther. 2016;7:269–78. doi: 10.1007/s13300-016-0163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Sanden S, Diels J, Pascal G, Nielsen AT. Bayesian Network Meta-analysis to Assess Relative Efficacy of Canagliflozin Versus Glucagon-like Peptide-1 Receptor Agonists in Dual and Triple Therapy in Patients With Type 2 Diabetes Mellitus. Poster – The 20th Annual Meeting of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Philadelphia, Pennsylvania: 2015. May 16-20, [Google Scholar]
- 92.Nauck MA, Del Prato S, Meier JJ, Durán-García S, Rohwedder K, Elze M, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: A randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011;34:2015–22. doi: 10.2337/dc11-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ridderstråle M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC EMPA-REG HH-SU trial investigators. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: A 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2:691–700. doi: 10.1016/S2213-8587(14)70120-2. [DOI] [PubMed] [Google Scholar]
- 94.Pacou M, Taieb V, Abrams K, Diels J, van Sanden S, Garg M, et al. Bayesian Network Meta-analysis to Assess the Relative Efficacy and Safety of Canagliflozin in Patients with Type 2 Diabetes Mellitus (t2dm) Inadequately Controlled on Metformin and Sulphonylurea (met+su). Poster – The 16th Annual European Congress of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Dublin, Ireland: 2013. Nov, [Google Scholar]
- 95.Häring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Woerle HJ, et al. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: A 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013;36:3396–404. doi: 10.2337/dc12-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jabbour SA, Hardy E, Sugg J, Parikh S. Study Group. Dapagliflozin is effective as add-on therapy to sitagliptin with or without metformin: A 24-week, multicenter, randomized, double-blind, placebo-controlled study. Diabetes Care. 2014;37:740–50. doi: 10.2337/dc13-0467. [DOI] [PubMed] [Google Scholar]
- 97.Kovacs CS, Seshiah V, Swallow R, Jones R, Rattunde H, Woerle HJ, et al. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: A 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2014;16:147–58. doi: 10.1111/dom.12188. [DOI] [PubMed] [Google Scholar]
- 98.Mathieu C, Ranetti AE, Li D, Ekholm E, Cook W, Hirshberg B, et al. Randomized, double-blind, phase 3 trial of triple therapy with dapagliflozin add-on to saxagliptin plus metformin in type 2 diabetes. Diabetes Care. 2015;38:2009–17. doi: 10.2337/dc15-0779. [DOI] [PubMed] [Google Scholar]
- 99.Matthaei S, Bowering K, Rohwedder K, Sugg J, Parikh S, Johnsson E. Study Group. Durability and tolerability of dapagliflozin over 52 weeks as add-on to metformin and sulphonylurea in type 2 diabetes. Diabetes Obes Metab. 2015;17:1075–84. doi: 10.1111/dom.12543. [DOI] [PubMed] [Google Scholar]
- 100.DeFronzo RA, Lewin A, Patel S, Liu D, Kaste R, Woerle HJ, et al. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care. 2015;38:384–93. doi: 10.2337/dc14-2364. [DOI] [PubMed] [Google Scholar]
- 101.Neal B, Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Ways K, et al. Efficacy and safety of canagliflozin, an inhibitor of sodium-glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38:403–11. doi: 10.2337/dc14-1237. [DOI] [PubMed] [Google Scholar]
- 102.Rosenstock J, Jelaska A, Frappin G, Salsali A, Kim G, Woerle HJ, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37:1815–23. doi: 10.2337/dc13-3055. [DOI] [PubMed] [Google Scholar]
- 103.Wilding JP, Woo V, Soler NG, Pahor A, Sugg J, Rohwedder K, et al. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: A randomized trial. Ann Intern Med. 2012;156:405–15. doi: 10.7326/0003-4819-156-6-201203200-00003. [DOI] [PubMed] [Google Scholar]
- 104.Rosenstock J, Jelaska A, Zeller C, Kim G, Broedl UC, Woerle HJ. EMPA-REG BASALTM Trial Investigators. Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: A 78-week randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2015;17:936–48. doi: 10.1111/dom.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Diels J, Neslusan C. Philadelphia, Pennsylvania: 2015. May 16-20, Comparative Persistency With Newer Agents Used to Treat Type 2 Diabetes in the United States: Canagliflozin Versus Dipeptidyl Peptidase-4 Inhibitors and glucagon-Like Peptide-1 Agonists. Poster – The 20th Annual Meeting of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) [Google Scholar]
- 106.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 107.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): A randomised controlled trial. Lancet. 2005;366:1279–89. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 108.Bolinder J, Ljunggren Ö, Johansson L, Wilding J, Langkilde AM, Sjöström CD, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab. 2014;16:159–69. doi: 10.1111/dom.12189. [DOI] [PubMed] [Google Scholar]
- 109.Bolinder J, Ljunggren Ö, Kullberg J, Johansson L, Wilding J, Langkilde AM, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97:1020–31. doi: 10.1210/jc.2011-2260. [DOI] [PubMed] [Google Scholar]
- 110.Blonde L, Stenlöf K, Fung A, Xie J, Canovatchel W, Meininger G. Effects of canagliflozin on body weight and body composition in patients with type 2 diabetes over 104 weeks. Postgrad Med. 2016;128:371–80. doi: 10.1080/00325481.2016.1169894. [DOI] [PubMed] [Google Scholar]
- 111.Inzucchi SE, Zinman B, Wanner C, Ferrari R, Fitchett D, Hantel S, et al. SGLT-2 inhibitors and cardiovascular risk: Proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res. 2015;12:90–100. doi: 10.1177/1479164114559852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Halimi S, Vergès B. Adverse effects and safety of SGLT-2 inhibitors. Diabetes Metab. 2014;40(6 Suppl 1):S28–34. doi: 10.1016/S1262-3636(14)72693-X. [DOI] [PubMed] [Google Scholar]
- 113.Chino Y, Samukawa Y, Sakai S, Nakai Y, Yamaguchi J, Nakanishi T, et al. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos. 2014;35:391–404. doi: 10.1002/bdd.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Davies MJ, Trujillo A, Vijapurkar U, Damaraju CV, Meininger G. Effect of canagliflozin on serum uric acid in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2015;17:426–9. doi: 10.1111/dom.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Baker WL, Smyth LR, Riche DM, Bourret EM, Chamberlin KW, White WB. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: A systematic review and meta-analysis. J Am Soc Hypertens. 2014;8:262–75-e9. doi: 10.1016/j.jash.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 116.Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: Rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495–502. doi: 10.1038/nrendo.2011.243. [DOI] [PubMed] [Google Scholar]
- 117.Majewski C, Bakris GL. Blood pressure reduction: An added benefit of sodium-glucose cotransporter 2 inhibitors in patients with type 2 diabetes. Diabetes Care. 2015;38:429–30. doi: 10.2337/dc14-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Patel AK, Fonseca V. Turning glucosuria into a therapy: Efficacy and safety with SGLT2 inhibitors. Curr Diab Rep. 2010;10:101–7. doi: 10.1007/s11892-010-0095-5. [DOI] [PubMed] [Google Scholar]
- 119.Nakatani Y, Kawabe A, Matsumura M, Aso Y, Yasu T, Banba N, et al. Effects of GLP-1 receptor agonists on heart rate and the autonomic nervous system using holter electrocardiography and power spectrum analysis of heart rate variability. Diabetes Care. 2016;39:e22–3. doi: 10.2337/dc15-1437. [DOI] [PubMed] [Google Scholar]
- 120.Njomnang Soh P, Vidal F, Huyghe E, Gourdy P, Halimi JM, Bouhanick B. Urinary and genital infections in patients with diabetes: How to diagnose and how to treat. Diabetes Metab. 2016;42:16–24. doi: 10.1016/j.diabet.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 121.Arakaki RF. Sodium-glucose cotransporter-2 inhibitors and genital and urinary tract infections in type 2 diabetes. Postgrad Med. 2016;128:409–17. doi: 10.1080/00325481.2016.1167570. [DOI] [PubMed] [Google Scholar]
- 122.Edwards S. Balanitis and balanoposthitis: A review. Genitourin Med. 1996;72:155–9. doi: 10.1136/sti.72.3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Geerlings S, Fonseca V, Castro-Diaz D, List J, Parikh S. Genital and urinary tract infections in diabetes: Impact of pharmacologically-induced glucosuria. Diabetes Res Clin Pract. 2014;103:373–81. doi: 10.1016/j.diabres.2013.12.052. [DOI] [PubMed] [Google Scholar]
- 124.Gonçalves B, Ferreira C, Alves CT, Henriques M, Azeredo J, Silva S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42:905–27. doi: 10.3109/1040841X.2015.1091805. [DOI] [PubMed] [Google Scholar]
- 125.Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: A systematic review and meta-analysis. Ann Intern Med. 2013;159:262–74. doi: 10.7326/0003-4819-159-4-201308200-00007. [DOI] [PubMed] [Google Scholar]
- 126.Ji L, Han P, Liu Y, Yang G, Dieu Van NK, Vijapurkar U, et al. Canagliflozin in Asian patients with type 2 diabetes on metformin alone or metformin in combination with sulphonylurea. Diabetes Obes Metab. 2015;17:23–31. doi: 10.1111/dom.12385. [DOI] [PubMed] [Google Scholar]
- 127.Nicolle LE, Capuano G, Fung A, Usiskin K. Urinary tract infection in randomized phase III studies of canagliflozin, a sodium glucose co-transporter 2 inhibitor. Postgrad Med. 2014;126:7–17. doi: 10.3810/pgm.2014.01.2720. [DOI] [PubMed] [Google Scholar]
- 128.Kalra S, Baruah MP, Sahay R. Medication counselling with sodium glucose transporter 2 inhibitor therapy. Indian J Endocrinol Metab. 2014;18:597–9. doi: 10.4103/2230-8210.139206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mendling W, Brasch J, Cornely OA, Effendy I, Friese K, Ginter-Hanselmayer G, et al. Guideline: Vulvovaginal candidosis (AWMF 015/072), S2k (excluding chronic mucocutaneous candidosis) Mycoses. 2015;58(Suppl 1):1–15. doi: 10.1111/myc.12292. [DOI] [PubMed] [Google Scholar]
- 130.Edwards SK, Bunker CB, Ziller F, van der Meijden WI. 2013 European guideline for the management of balanoposthitis. Int J STD AIDS. 2014;25:615–26. doi: 10.1177/0956462414533099. [DOI] [PubMed] [Google Scholar]
- 131.Donders GG, Bellen G, Mendling W. Management of recurrent vulvo-vaginal candidosis as a chronic illness. Gynecol Obstet Invest. 2010;70:306–21. doi: 10.1159/000314022. [DOI] [PubMed] [Google Scholar]
- 132.Kalra S, Chawla A. Diabetes and balanoposthitis. J Pak Med Assoc. 2016;66:1039–41. [PubMed] [Google Scholar]
- 133.EMA Confirms Recommendations to Minimise Ketoacidosis Risk with SGLT2 Inhibitors for Diabetes. [Last accessed on 2016 Jul 18]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2016/02/news_detail_002477.jsp .
- 134.FDA Revises Labels of SGLT2 Inhibitors for Diabetes to Include Warnings about Too Much Acid in the Blood and Serious Urinary Tract Infections. [Last accessed on 2016 Jul 18]. Available from: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM475487.pdf .
- 135.FDA Drug Safety Communication: Interim Clinical Trial Results Find Increased Risk of Leg and Foot Amputations, Mostly Affecting the Toes, with the Diabetes Medicine Canagliflozin (Invokana, Invokamet); FDA to Investigate. [Last accessed on 2016 Jul 18]. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm500965.htm .
- 136.EMA Reviews Diabetes Medicine Canagliflozin. Review Follows Data on Toe Amputations in Ongoing Study. [Last accessed on 2016 Jul 18]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/SGLT2_inhibitors_Canagliflozin_20/Procedure_started/WC500204901.pdf .
- 137.Taylor SI, Blau JE, Rother KI. Possible adverse effects of SGLT2 inhibitors on bone. Lancet Diabetes Endocrinol. 2015;3:8–10. doi: 10.1016/S2213-8587(14)70227-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962–71. doi: 10.1038/ki.2013.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.EMEA – Bone Fractures with Canagliflozin. [Last accessed on 2016 Jul 18]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/SGLT2_inhibitors__20/European_Commission_final_decision/WC500206511.pdf .
- 140.Canagliflozin – Review of Bone Safety Data. FDA Briefing Document. NDA 204042. Endocrinologic and Metabolic Drugs Advisory Committee Meeting. 2013. Jan 10, [Last accessed on 2016 Oct 20]. Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM334551.pdf .
- 141.Tang HL, Li DD, Zhang JJ, Hsu YH, Wang TS, Zhai SD, et al. Lack of evidence for a harmful effect of sodium-glucose co-transporter 2 (SGLT2) inhibitors on fracture risk among type 2 diabetes patients: A network and cumulative meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2016;18:1199–206. doi: 10.1111/dom.12742. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 142.Lin HW, Tseng CH. A review on the relationship between SGLT2 inhibitors and cancer. Int J Endocrinol 2014. 2014:719578. doi: 10.1155/2014/719578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Obermeier M, Yao M, Khanna A, Koplowitz B, Zhu M, Li W, et al. In vitro characterization and pharmacokinetics of dapagliflozin (BMS-512148), a potent sodium-glucose cotransporter type II inhibitor, in animals and humans. Drug Metab Dispos. 2010;38:405–14. doi: 10.1124/dmd.109.029165. [DOI] [PubMed] [Google Scholar]
- 144.Bode B, Stenlöf K, Harris S, Sullivan D, Fung A, Usiskin K, et al. Long-term efficacy and safety of canagliflozin over 104 weeks in patients aged 55-80 years with type 2 diabetes. Diabetes Obes Metab. 2015;17:294–303. doi: 10.1111/dom.12428. [DOI] [PubMed] [Google Scholar]
- 145.Leiter LA, Cefalu WT, de Bruin TW, Gause-Nilsson I, Sugg J, Parikh SJ. Dapagliflozin added to usual care in individuals with type 2 diabetes mellitus with preexisting cardiovascular disease: A 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. J Am Geriatr Soc. 2014;62:1252–62. doi: 10.1111/jgs.12881. [DOI] [PubMed] [Google Scholar]
- 146.Sarashina A, Ueki K, Sasaki T, Tanaka Y, Koiwai K, Sakamoto W, et al. Effect of renal impairment on the pharmacokinetics, pharmacodynamics, and safety of empagliflozin, a sodium glucose cotransporter 2 inhibitor, in Japanese patients with type 2 diabetes mellitus. Clin Ther. 2014;36:1606–15. doi: 10.1016/j.clinthera.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 147.National Institute of Diabetes and Digestive and Kidney Diseases. Glomerular Filtration Rate (GFR) Calculators. [Last accessed on 2016 Jun 29]. http://www.niddk.nih.gov/health-information/health-communication-programs/nkdep/labevaluation/gfr-calculators/Pages/gfr-calculators.aspx .
- 148.Botev R, Mallie JP, Couchoud C, Schuck O, Fauvel JP, Wetzels JF, et al. Estimating glomerular filtration rate: Cockcroft-Gault and Modification of Diet in Renal Disease formulas compared to renal inulin clearance. Clin J Am Soc Nephrol. 2009;4:899–906. doi: 10.2215/CJN.05371008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Canagliflozin (invokana). A “me-too” of the dangerous dapagliflozin. Prescrire Int. 2015;24:33–5. [PubMed] [Google Scholar]
- 150.Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, et al. Intensive glycemic control and the prevention of cardiovascular events: Implications of the ACCORD, ADVANCE, and VA diabetes trials: A position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care. 2009;32:187–92. doi: 10.2337/dc08-9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.FDA Briefing Document-Dapagliflozin. NDA 202293. Advisory Committee Meeting. 2013. Dec 12, [Last accessed on 2016 Jul 18]. Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM378076.pdf .
- 152.Monami M, Dicembrini I, Mannucci E. Effects of SGLT-2 inhibitors on mortality and cardiovascular events: A comprehensive meta-analysis of randomized controlled trials. Acta Diabetol. 2016 doi: 10.1007/s00592-016-0892-7. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 153.Nassar AA, Childs RD, Boyle ME, Jameson KA, Fowke M, Waters KR, et al. Diabetes in the desert: What do patients know about the heat? J Diabetes Sci Technol. 2010;4:1156–63. doi: 10.1177/193229681000400514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.John M, Violante R, Deerochanawong C, Vijapurkar U, Canovatchel W, Hamilton G. Efficacy and Safety of Canagliflozin in Patients With Type 2 Diabetes Mellitus Living in Hot Climates Poster – 51st Annual Meeting of the European Association for The Study of Diabetes (EASD) Stockholm, Sweden: 2015. Sep 14-18, [Google Scholar]
- 155.Kalra S, Aamir AH, Raza A, Das AK, Azad Khan AK, Shrestha D, et al. Place of sulfonylureas in the management of type 2 diabetes mellitus in South Asia: A consensus statement. Indian J Endocrinol Metab. 2015;19:577–96. doi: 10.4103/2230-8210.163171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wan Seman WJ, Kori N, Rajoo S, Othman H, Mohd Noor N, Wahab NA, et al. Switching from sulphonylurea to a sodium-glucose cotransporter2 inhibitor in the fasting month of Ramadan is associated with a reduction in hypoglycaemia. Diabetes Obes Metab. 2016;18:628–32. doi: 10.1111/dom.12649. [DOI] [PubMed] [Google Scholar]
- 157.Kelwade J, Sethi BK, Vaseem A, Nagesh VS. Sodium glucose co transporter 2 inhibitors and Ramadan: Another string to the bow. Indian J Endocrinol Metab. 2014;18:874–5. doi: 10.4103/2230-8210.141397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Niazi AK, Kalra S. Patient centred care in diabetology: An Islamic perspective from South Asia. J Diabetes Metab Disord. 2012;11:30. doi: 10.1186/2251-6581-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kalra S, Bajaj S, Gupta Y, Agarwal P, Singh SK, Julka S, et al. Fasts, feasts and festivals in diabetes-1: Glycemic management during Hindu fasts. Indian J Endocrinol Metab. 2015;19:198–203. doi: 10.4103/2230-8210.149314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bailey TS, Grunberger G, Bode BW, Handelsman Y, Hirsch IB, Jovanovic L, et al. American Association of Clinical Endocrinologists and American College of Endocrinology 2016 outpatient glucose monitoring consensus statement. Endocr Pract. 2016;22:231–61. doi: 10.4158/EP151124.CS. [DOI] [PubMed] [Google Scholar]
- 161.Standards of medical care in diabetes-2016: Summary of revisions. Diabetes care. 2016;39(Suppl 1):S4–5. doi: 10.2337/dc16-S003. [DOI] [PubMed] [Google Scholar]
- 162.QRISK®2-2016 Risk Calculator. [Last accessed on 2016 Jul 11]. Available from: https://www.qrisk.org/
- 163.Kalra S. Health risk calculators for Pakistan. J Pak Med Assoc. 2016;66:1669–70. [PubMed] [Google Scholar]
- 164.QDiabetes®(Heart failure) – 2015 Risk Calculator. [Last accessed on 2016 Jul 15]. Available from: http://www.qdiabetes.org/heart-failure .