Abstract
Dysregulated catecholamine signaling has long been implicated in drug abuse. Although much is known about adaptations following chronic drug administration, little work has investigated how a single drug exposure paired with withdrawal influences catecholamine signaling in vivo. We used fast-scan cyclic voltammetry in freely moving rats to measure real-time catecholamine overflow during acute morphine exposure and naloxone-precipitated withdrawal in two regions associated with the addiction cycle: the dopamine-dense nucleus accumbens (NAc) and norepinephrine-rich ventral bed nucleus of the stria terminalis (vBNST). We compared dopamine transients in the NAc with norepinephrine concentration changes in the vBNST, and correlated release with specific withdrawal-related behaviors. Morphine increased dopamine transients in the NAc, but did not elicit norepinephrine responses in the vBNST. Conversely, dopamine output was decreased during withdrawal, while norepinephrine was released in the vBNST during specific withdrawal symptoms. Both norepinephrine and withdrawal symptoms could be elicited in the absence of morphine by administering naloxone with an α2 antagonist. The data support reciprocal roles for dopamine and norepinephrine signaling during drug exposure and withdrawal. The data also support the allostasis model and show that negative-reinforcement may begin working after a single exposure/withdrawal episode.
INTRODUCTION
Drug exposure causes lasting neural adaptations that increase the risk for relapse long after drug cessation (Nestler, 2001), and considerable evidence indicates forebrain catecholamine circuits become dysregulated during the development of addiction (Koob and Volkow, 2010). In the allostasis model, drug abuse develops after a shift from positively to negatively reinforced drug use, and adaptations in catecholaminergic signaling may contribute to this phenomenon (Koob and Volkow, 2010). Drugs of abuse have diverse actions on dopaminergic signaling in the ventral tegmental area (VTA) and its afferents (Juarez and Han, 2016), where dopamine is thought to aid in associative memory formation and drive cue-induced drug seeking (Berke and Hyman, 2000; Everitt and Robbins, 2005; Hyman et al, 2006). Action potentials from the VTA drive transient dopamine concentration fluctuations in the nucleus accumbens (NAc) (Sombers et al, 2009) that increase following drug administration and can potentiate a drug's positively reinforcing properties (Covey et al, 2014). However, NAc dopamine may also play a role in the negatively reinforced component of drug abuse, since dopamine overflow is suppressed during noxious and aversive stimuli (Park et al, 2015; Roitman et al, 2008; Twining et al, 2015). Although basal dopamine levels decrease during withdrawal (Pothos et al, 1991; Weiss et al, 1996), previous work does not address its effect on phasic dopamine concentrations. Bidirectional changes in dopamine signaling likely contribute to drug use (Koob and Volkow, 2010); therefore, how dopamine signals during both drug exposure and withdrawal is an important area of investigation.
Norepinephrine signaling also plays a role in drug reward (Olson et al, 2006) and withdrawal aversion (Delfs et al, 2000). Suppression of noradrenergic signaling blocks stress-induced reinstatement (Erb et al, 2000; Leri et al, 2002; Shaham et al, 2000), and treatment with the adrenergic agonist clonidine promotes heroin-abstinence in human addicts (Kowalczyk et al, 2015). Although few studies emphasize the noradrenergic component of drug use, norepinephrine in the bed nucleus of the stria terminalis (BNST) has been clearly implicated in drug withdrawal (Aston-Jones et al, 1999). The ventral BNST (vBNST) receives dense innervation from the nucleus of the solitary tract (NTS) (Forray and Gysling, 2004), the source of norepinephrine critical for withdrawal aversion (Delfs et al, 2000), and chronic morphine treatment increases basal norepinephrine in the BNST (Fuentealba et al, 2000). However, no work addresses the effect of acute drug exposure or withdrawal on phasic release. Moreover, stress-dependent adaptations in vBNST noradrenergic signaling arise following morphine-dependence (Fox et al, 2015; McElligott et al, 2013), yet the source of this plasticity is unknown.
Many studies focus on chronic drug administration (Harris and Aston-Jones, 1994; Hemby et al, 1995; Kaufling and Aston-Jones, 2015; Mazei-Robison and Nestler, 2012); however, it is unknown how drug exposure paired with withdrawal alters catecholamine signaling in regions associated with the addiction cycle. Since appetitive and aversive stimuli elicit opposing responses from catecholamines (Park et al, 2012; Roitman et al, 2008; Twining et al, 2015), we hypothesized dopamine and norepinephrine signal reciprocally during drug exposure and withdrawal. We used voltammetry to measure catecholamine release in freely moving animals exposed to morphine and naloxone-precipitated withdrawal. During drug exposure, dopamine signaling increased in the NAc but morphine did not elicit norepinephrine in the vBNST. Conversely, drug withdrawal produced vBNST norepinephrine release that coincided with specific withdrawal symptoms. Finally, vBNST norepinephrine release, but not NAc dopamine, was attenuated after treatment. These data provide a real-time view of catecholamine signaling during drug exposure and withdrawal, and lend insight to how catecholamine circuits may become dysregulated after drug use.
MATERIALS AND METHODS
Additional details are described in Supplementary Methods.
Animal Care
All experiments were performed in accordance with the Institutional Animal Care and Use Committee guidelines of the University of North Carolina at Chapel Hill (UNC). Sprague-Dawley rats (males, 270–350 g; Charles River, Wilmington, MA) were given food and water ad libitum and pair-housed in UNC animal facilities on a 12 : 12-h light : dark cycle until surgery. Animals with misplaced electrodes, as well as animals that exhibited mixed pharmacological response, were excluded from the study (see Supplementary Figure S1). A total of 14 animals were included for dopamine measurements (7 morphine–naloxone, 7 vehicle–naloxone), and 29 for norepinephrine measurements (7 morphine–naloxone, 7 vehicle–naloxone, 6 morphine–naloxone +idazoxan, 6 vehicle–naloxone+idazoxan, 3 spontaneous withdrawal). Two separate groups of animals were used to examine the acute effects of morphine on evoked dopamine (n=4), and idazoxan on dopamine transients (n=3). All measurements took place during the light cycle.
Sterotaxic Surgery
Animals were anesthetized with isofluorane (4% induction, 1.5% maintenance) and affixed in a stereotaxic frame (Kopf Instruments). The scalp was removed and holes were drilled to implant guide cannulas in the right hemisphere (BASi, West Lafayette, IN) above the NAc shell (AP: +1.7 mm, ML: +0.8 mm) or vBNST (AP: 0 mm, ML: +1.2 mm), referenced from bregma and based on the atlas of Paxinos and Watson. An additional guide cannula was implanted in the left hemisphere for insertion of an Ag/AgCl reference electrode on the day of recording. A bipolar stimulating electrode (Plastics One, Roanoke, VA) was targeted to the right VTA/ventral noradrenergic bundle (VNB; AP −5.2 mm, ML +1.0, DV −8.2 mm), and a dental cement skull-cap was secured with jeweler's screws. Animals were subsequently given 300 mg/kg acetaminophen p.o., singly housed and allowed to recover for 3 days before making measurements. We monitored animal recovery and did not make measurements in any animals appearing to be in pain (eg, ruffled fur, increased irritability).
Voltammetric Catecholamine Measurements
Fast scan cyclic voltammetry measurements of dopamine and norepinephrine were performed in awake animals as described previously (Park et al, 2013). Briefly, a fresh carbon-fiber electrode (~100 μm active length) was lowered into the NAc shell or vBNST through the guide cannula with a custom-built micromanipulator. A triangular waveform (−0.4 to +1.3 V, 400 V/s) was applied to the electrode every 100 ms to oxidize and reduce catecholamines using HDCV for data acquisition and analysis. After electrode placement, we recorded baseline signaling for ~10 min before giving 10 mg/kg morphine sulfate s.c. (Sigma-Aldrich, St Louis, MO) or 1 ml/kg vehicle (saline 0.9%). Four hours later, we administered 1 mg/kg naloxone HCl s.c. (Sigma-Aldrich) and scored animals for somatic indices of withdrawal (Schulteis et al, 1999) over 20 min. In a subset of animals, 5 mg/kg idazoxan was given with naloxone to elicit norepinephrine overflow. One hour after naloxone administration, animals were anesthetized with urethane (1.5 g/kg), and the VTA/VNB was electrically stimulated using constant current isolators (Neurologs, model NL800) to obtain voltammograms for principal component analysis (PCA)(Rodeberg et al, 2015). We extracted dopamine currents using PCA and converted current to concentration using an averaged in vitro calibration factor (10 nA/μM). Only dopamine transients >3 times the noise in the traces obtained by PCA were considered to be dopamine transients, and we measured increased dopamine transient frequency during the first hour of morphine administration. In awake animals, voltammetric peaks for norepinephrine release in the vBNST were broader than expected. We suspect contribution from a noradrenergic metabolite during withdrawal because these features were mimicked in anesthetized rats with long stimulations, and were eliminated following inhibition of monoamine oxidase with clorgyline. Norepinephrine currents were extracted with PCA; however, the extent to which the metabolite component influences in vitro calibration factors is unknown. For this reason, we report extracted norepinephrine currents, but not concentration in awake animals. When applicable, an averaged in vitro calibration factor (6 nA/μM) was used to convert norepinephrine current to concentration in anesthetized animals.
vBNST Pharmacology
At the end of awake-animal recordings, we confirmed that the signal in the vBNST was due to norepinephrine and not dopamine by measuring evoked release after 2 mg/kg s-(−)-raclopride tartrate (D2 antagonist; Sigma-Aldrich) and 5 mg/kg idaxoxan HCl (α2 antagonist; Sigma Aldrich). Only signals that responded to idazoxan but not raclopride were classified as norepinephrine release. Signals with mixed pharmacological response were excluded as in Supplementary Figure S1.
Somatic Withdrawal Signs
Behavioral correlates of opiate withdrawal were measured during the first 20 min of precipitated withdrawal and assigned a global withdrawal score as described previously and in detail in Supplementary Methods (McElligott et al, 2013). We manually assigned time-stamps to each behavior and looked for the presence or absence of dopamine transients or norepinephrine overflow during the withdrawal signs. Somatic withdrawal signs were either considered absent, present, or present with catecholamine signaling.
Statistics
All statistical tests were performed in Graph Pad Prism. Two-way, repeated measures analysis of variance (ANOVA) with Bonferroni post hoc were used to assess differences in release event frequency and concentration under varying drug conditions. An unpaired Welch's corrected t-test was used to determine significant differences between evoked norepinephrine concentrations after withdrawal due to significant differences in the variance between morphine- and vehicle-treated groups.
RESULTS
Morphine-Exposure Increases Dopaminergic Transmission in the NAc
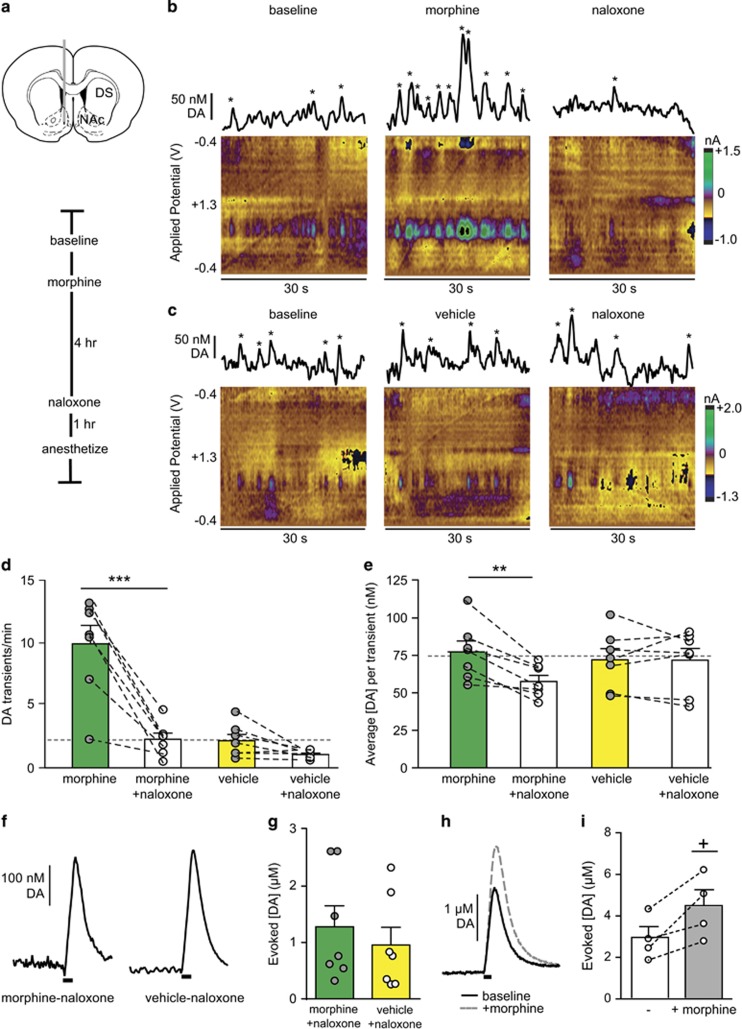
Drugs of abuse increase dopaminergic transmission in the NAc (Cheer et al, 2007; Daberkow et al, 2013; Di Chiara and Imperato, 1988; Phillips et al, 2003; Volkow et al, 2007), which contributes to their acutely reinforcing properties. We used a dose of morphine that, when paired with precipitated withdrawal, generates rapid dependence in rats (Schulteis et al, 1999). To confirm that morphine elevates dopamine in the NAc of freely moving rats (Vander Weele et al, 2014), we used voltammetry to record dopamine transients in locations exhibiting spontaneous activity (~2 transients per minute; schematic of placement and time line in Figure 1a, representative traces and color plots in Figure 1b and c). We found that morphine increased spontaneous dopamine transient frequency in the NAc (10 mg/kg s.c.) relative to saline (vehicle, 1 ml/kg s.c.) (9.9±1.5 vs 2.1±0.5 transients per minute, morphine vs vehicle, N=7, respectively, t=4.976, P=0.0016; Figure 1d), but the average concentration per transient was similar under both conditions (morphine: 77.3±7 vs vehicle: 72.1±7 nM, N=7, respectively; Figure 1e).
Figure 1.
Dopamine efflux in the nucleus accumbens (NAc) varies with morphine exposure and withdrawal. (a) Schematic of electrode placement and experimental timeline. (b) Representative dopamine (DA) transients at baseline, after morphine, and after naloxone. Principal component analysis identified concentrations marked with asterisks as DA transients. (c) Representative DA transients at baseline, after vehicle, and after naloxone. (d) Transient frequency and (e) average DA transient concentration under morphine or vehicle and subsequent naloxone. Average frequency and concentration of dopamine transients at baseline are indicated by the gray dashed lines. (f) Representative evoked [DA] after treatment in morphine–naloxone or vehicle–naloxone animals and (g) data from all subjects. (h) Representative evoked [DA] in naïve animals at baseline (solid line) and after morphine (hashed line) and (i) data from all subjects. Bar graphs show average±SEM with individual subjects overlaid. **P<0.01, ***P<0.001, two-way repeated measures ANOVA, Bonferroni post hoc. +, P<0.05, paired T-test. DS, dorsal striatum.
Naloxone-Precipitated Withdrawal Decreases Dopaminergic Transmission in the NAc
We next examined the impact of drug withdrawal by administering naloxone (1 mg/kg s.c.) 4 h after initial morphine (Fox et al, 2015; McElligott et al, 2013; Schulteis et al, 1999). We recorded dopamine transients over 20 min during the peak of somatic withdrawal signs and found dopamine transient frequency decreased in animals undergoing withdrawal relative to initial treatment (9.9±1.5 vs 2.3±0.5 transients per minute, morphine vs morphine–naloxone; 2.1±0.5 vs 1.1±0.1 transients per minute, vehicle vs vehicle–naloxone, N=7, respectively; Figure 1d). Post hoc analysis revealed a significant decrease in dopamine transient frequency between morphine and morphine–naloxone (two-way repeated measures ANOVA, morphine treatment group × naloxone interaction F(1,12)=22.60, P=0.0005; morphine treatment F(1,12)=23.06, P=0.0004; naloxone treatment F(1,12)=40.30, P<0.0001, Bonferroni post hoc, P<0.001) but not between vehicle and vehicle–naloxone (P>0.05). Moreover, average dopamine transient concentrations decreased during withdrawal that was not solely due to naloxone (morphine–naloxone: 57.6±4 vs vehicle–naloxone: 71.9±8 nM, N=7, respectively. Two-way repeated measures ANOVA; morphine treatment group × naloxone interaction F(1,12)=9.667, P=0.0090, naloxone F(1,12)=10.3, P=0.0075, Bonferonni post hoc, P<0.01; Figure 1e), reflecting decreased dopaminergic output in the drug-withdrawn group. To determine if decreased dopamine concentrations in morphine–naloxone animals were due to adaptations in releasable dopamine, we anesthetized the animals after treatment and electrically stimulated the VTA. We found equivalent evoked dopamine in morphine–naloxone and vehicle–naloxone-treated animals (1.28±.37 vs 0.96±0.31 μM, N=7, respectively, t=0.6634, P=0.5196; Figure 1f and g). In a separate group of rats, we assayed the effect of morphine alone on electrically evoked dopamine release. Morphine increased evoked dopamine concentrations (baseline vs morphine: 2.9±1.5 vs 4.4±0.8 μM, N=4, P=0.04, paired T-test; Figure 1h and i). Acute exposure/withdrawal did not elicit persistent adaptations, in agreement with our previous study showing dopamine tissue content is unchanged in the NAc after repeated morphine/naloxone treatment (McElligott et al, 2013).
Morphine Withdrawal, but not Exposure, Elicits Norepinephrine in the vBNST
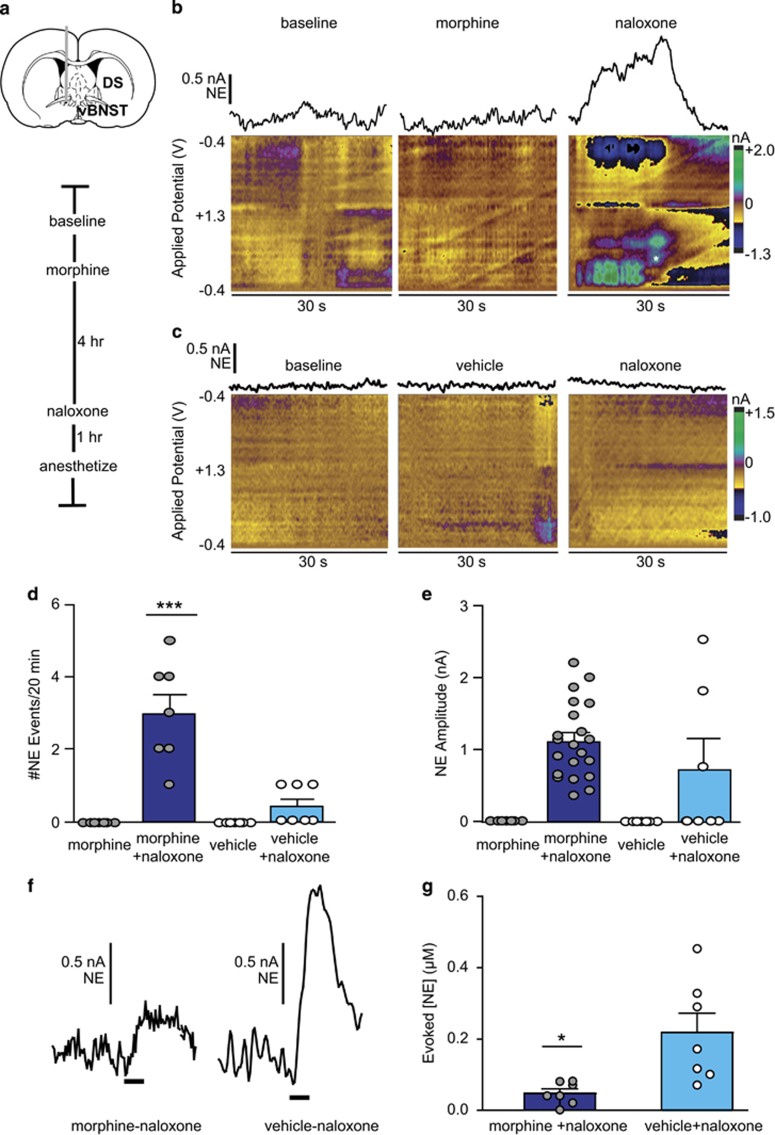
Norepinephrine's role in drug addiction is often overshadowed by dopamine, despite evidence that morphine reward may be contingent on brainstem norepinephrine synthesis (Olson et al, 2006). Additionally, opiate-withdrawal aversion requires forebrain norepinephrine (Delfs et al, 2000), and morphine-dependence alters noradrenergic synaptic function in the vBNST (Fox et al, 2015; McElligott et al, 2013). We thus measured norepinephrine release in the vBNST during morphine exposure and withdrawal (schematic in Figure 2a). Unlike dopamine, norepinephrine concentrations in the vBNST do not fluctuate spontaneously in animals at rest, and morphine did not elicit norepinephrine overflow in the vBNST (examples in Figure 2b and c). However, we observed broad norepinephrine release events during naloxone-precipitated withdrawal (eg, Figure 2b, d and e) that did not occur without naloxone (Supplementary Figure S2). Norepinephrine release persisted for tens of seconds, in contrast to brief (~1 s) dopamine transients recorded in the NAc, in a manner similar to previous studies (Park et al, 2012, 2013). Voltammetric peaks for norepinephrine were also broader than expected, and we suspect contribution from a noradrenergic metabolite during withdrawal (Supplementary Figure S3). Naloxone elicited norepinephrine release in some vehicle-treated animals; however, the occurrence of norepinephrine release events was greater in animals undergoing morphine withdrawal relative to vehicle–naloxone animals (morphine–naloxone: 3.0±0.5 vs vehicle–naloxone: 0.4±0.2 events per animal, N=7, respectively; two-way repeated measures ANOVA: morphine-treatment group F(1,12)=11.57, treatment group × naloxone interaction F(1,12)=11.57, naloxone F(1,12)=20.25, Bonferroni post hoc, P<0.001; Figure 2d). Norepinephrine release amplitudes did not differ between groups (morphine-naloxone: 1.1±0.1 nA, N=21 events vs vehicle-naloxone: 1.7±0.5 nA N=3 events; Figure 2e). We next assayed releasable norepinephrine by electrically stimulating the VNB and found significant attenuation in morphine withdrawn animals (0.047±0.011 vs 0.218±0.053 μM, N=7; t=3.115, P=0.0207; Figure 2f and g). This finding aligns with our previous study demonstrating reduced norepinephrine tissue content after repeated naloxone-precipitated withdrawal (McElligott et al, 2013). Moreover, this was not an effect of morphine alone, as morphine does not alter evoked norepinephrine in anesthetized animals (McElligott et al, 2013), and animals that underwent spontaneous withdrawal (without naloxone) had similar release compared to vehicle-naloxone animals (Supplementary Figure S2).
Figure 2.
Naloxone-precipitated withdrawal produces norepinephrine overflow in the ventral bed nucleus of the stria terminalis (vBNST). (a) Schematic of electrode placement and experimental timeline. (b, c) Representative norepinephrine (NE) response at baseline, after morphine or vehicle, and after naloxone. NE current is isolated from corresponding color plots using principal component analysis. Asterisk suggests oxidation of metabolite. (d) Total NE release events during morphine/vehicle and subsequent naloxone. ***P<0.001, two-way repeated measures ANOVA with Bonferroni post hoc. (e) Release amplitude during morphine/vehicle and subsequent naloxone. Bars are average ±SEM with individual events overlaid. (f) Representative evoked NE after treatment in morphine–naloxone and vehicle–naloxone animals and (g) data from all subjects. *P<0.05, Welch's corrected unpaired t-test. DS, dorsal striatum.
Catecholamine Signaling Differs During Expression of Withdrawal-Related Behaviors
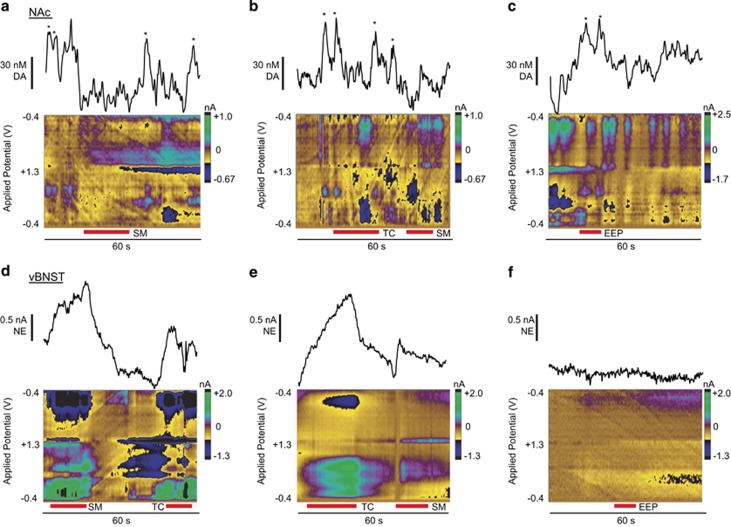
Somatic withdrawal signs like teeth-chattering and eye-twitches are a hallmark of opiate withdrawal in rats (Schulteis et al, 1999). Naloxone treatment produced somatic withdrawal behaviors and global withdrawal scores similar to our previous reports (Fox et al, 2015; McElligott et al, 2013), and scores were significantly higher in morphine-withdrawn animals (Supplementary Figure S5, two-way ANOVA, Bonferroni Post hoc: main effect of morphine F(1,12)=57.65, P<0.0001. DA: morphine–naloxone, 5.7±1.0 vs vehicle–naloxone, 2.0±0.5; NE: morphine–naloxone, 7.2±0.5 vs vehicle–naloxone, 1.0±0.4 N=7, respectively). To determine if catecholamine overflow was associated with withdrawal symptoms, we recorded the timing of somatic withdrawal behaviors and looked for concurrent dopamine or norepinephrine release. Morphine withdrawal normalized the frequency of dopamine transients, and in some cases, transients appeared to pause during certain behaviors (eg, swallowing movements, SM; example in Figure 3a). This effect varied between subjects (example of teeth chattering, TC; Figure 3b). Dopamine release was loosely associated with spontaneous erection/ejaculation/penile grooming (EEP) in the three animals exhibiting the behavior (example in Figure 3c).
Figure 3.
Somatic withdrawal signs produce variable catecholamine release in the nucleus accumbens (NAc) and ventral bed nucleus of the stria terminalis (vBNST). (a–c) Dopamine (DA) transients during swallowing movements (SM), teeth-chattering (TC), and spontaneous erection/ejaculation/penile grooming (EEP). Asterisks denote DA transients extracted with principal component analysis from the corresponding color plot. (d–f) Norepinephrine (NE) release occurs during SM and TC and is absent during EEP as extracted from the corresponding color plot with principal component analysis. Red bars beneath color plots denote duration of withdrawal behaviors.
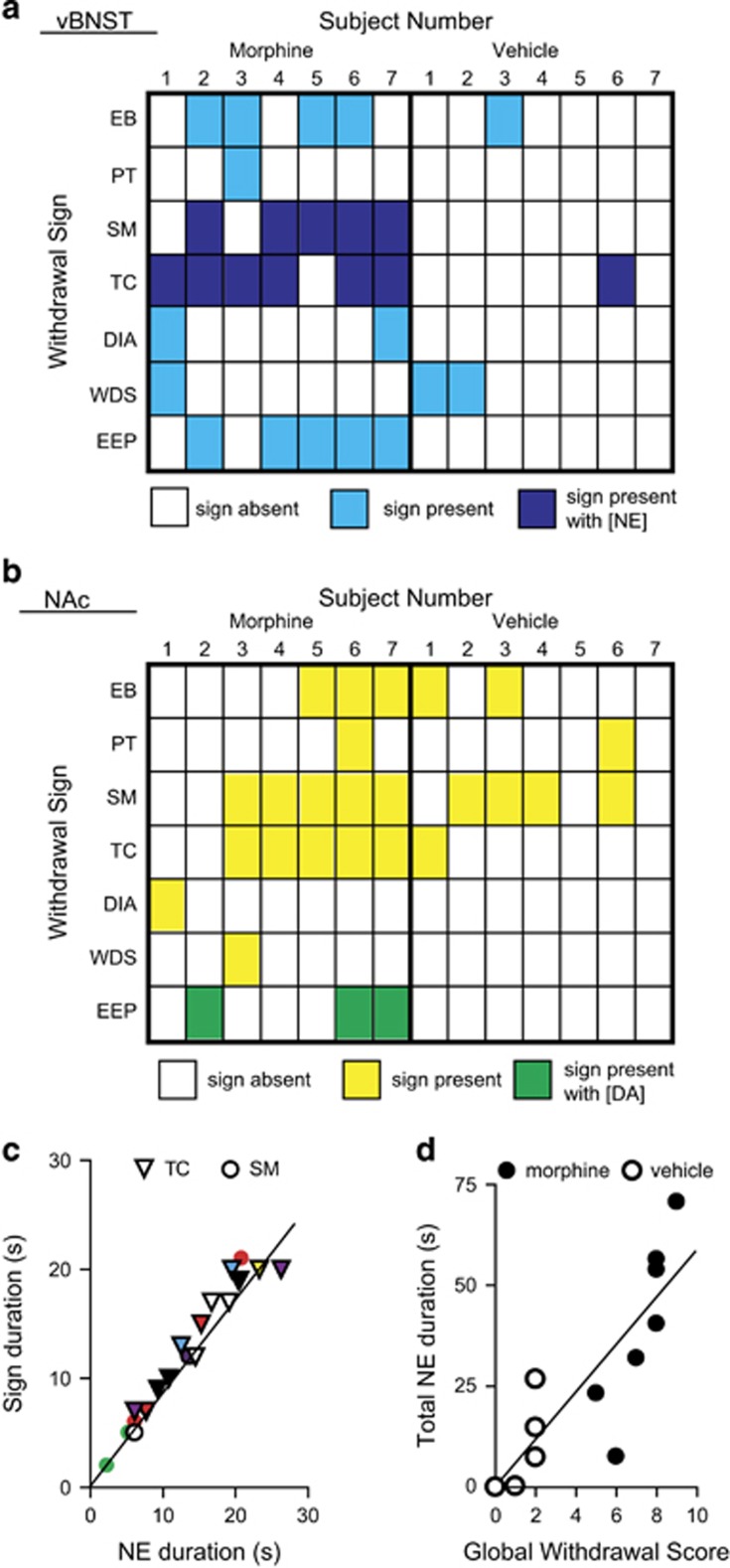
Norepinephrine release occurred with SM and TC in morphine-withdrawn animals (examples in Figure 3d and e), and in one vehicle-naloxone animal, but not during EEP (example in Figure 3f). When we compared the incidence of somatic withdrawal behaviors with catecholamine release, we found that every TC or SM occurred with norepinephrine release (Figure 4a), but was not time-locked to dopamine transients (Figure 4b). For this reason, we chose to further investigate the link between somatic withdrawal behaviors of TC/SM and norepinephrine release. We compared the duration of norepinephrine overflow with the duration of TC and SM and found a linear relationship between TC/SM duration and norepinephrine release (slope: 0.88±0.05, r2=0.95; Figure 4c), that is, when SM initiated, we measured increased norepinephrine; when SM terminated, so did the norepinephrine signal. We next compared the total duration of norepinephrine release in individual subjects vs their global withdrawal scores and found a linear correlation (slope: 5.8±0.9, r2=0.76; Figure 4d).
Figure 4.
Catecholamine signaling coincides with specific withdrawal behaviors. (a) Heat map illustrating withdrawal signs coinciding with norepinephrine signaling in individual subjects. White, sign absent; light blue, sign present; dark blue, sign occurs with norepinephrine (NE) overflow in the ventral bed nucleus of the stria terminalis (vBNST). (b) Heat map illustrating withdrawal signs coinciding with dopamine (DA) signaling. White, sign absent; yellow, sign present; green, sign occurs with DA transients. (c) Duration of teeth-chattering (TC, triangles) and swallowing movements (SM, circles) as a function of coincident NE overflow. Individual subjects are represented by different colors. (d) Total NE duration in individual subjects over 20 min withdrawal period vs global withdrawal score in morphine–naloxone (black) and vehicle–naloxone (white) animals. Abbreviations: EB, excessive eye blinks; PT, ptosis; SM, swallowing movements; TC, teeth-chattering; DIA, diarrhea; WDS, wet dog shakes; EEP, spontaneous erection/ejaculation/penile grooming.
Systemic α2 Antagonism with Naloxone Produced Withdrawal Signs and Norepinephrine in the Absence of Morphine
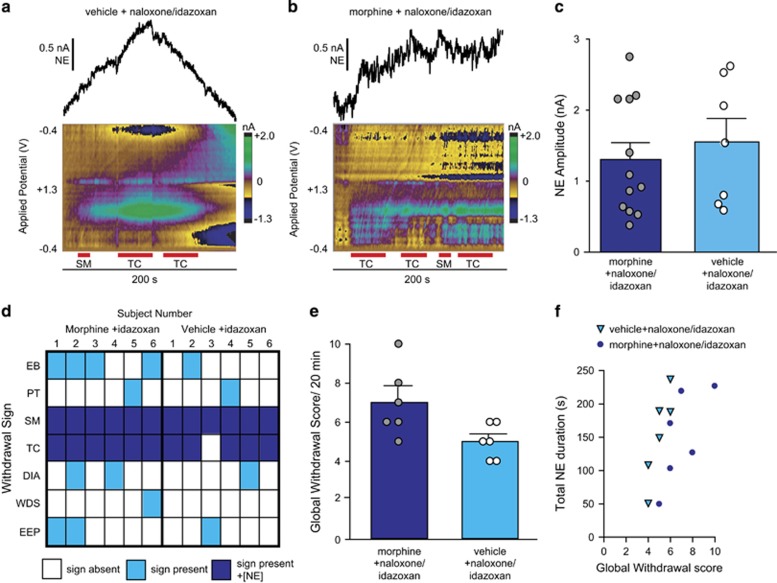
To strengthen the relationship between norepinephrine overflow and TC/SM, we next administered the α2 antagonist idazoxan with naloxone to elicit norepinephrine overflow in drug-naïve animals (example in Figure 5a). Systemic α2 antagonism elicited norepinephrine in vehicle–naloxone animals, and did not significantly alter the magnitude of norepinephrine release in animals given idazoxan during naloxone-precipitated morphine withdrawal (example in Figure 5b and all data in Figure 5c). Accompanying norepinephrine release in vehicle–naloxone/idazoxan-treated animals were increased withdrawal signs (Figure 5d) and global withdrawal scores (Figure 5e). When we compared total norepinephrine duration with global withdrawal scores, there was not a strong linear relationship (slope: 25.0±2.5, r2=0.31; Figure 5f). However, when we compared the duration of overflow only in vehicle–naloxone/idazoxan animals, we found a linear correlation (slope: 66.6±16.5, r2=0.80).
Figure 5.
Idazoxan enhances naloxone-precipitated withdrawal signs. (a, b) Representative color plots and extracted norepinephrine (NE) currents in vehicle (a) and morphine (b) animals treated with naloxone+ 5 mg/kg idazoxan. Duration of withdrawal behaviors (TC, teeth-chattering; SM, swallowing movements) is denoted by the red bars beneath the color plots. (c) Average±SEM amplitude of NE release in morphine (dark blue) and vehicle (light blue) animals during naloxone+idazoxan administration with individual data points overlaid. (d) Heat map illustrating withdrawal signs in individual subjects that coincide with NE overflow. White, sign absent; light blue, sign present; dark blue, sign present with NE overflow. (e) Global withdrawal score in morphine (dark blue) and vehicle (light blue) treated animals given naloxone+idazoxan. (f) Total NE duration in individual subjects (triangles, vehicle+naloxone/idazoxan; circles, morphine+naloxone/idazoxan) over the 20 min withdrawal period vs global withdrawal score. Abbreviations: EB, excessive eye blinks; PT, ptosis; SM, swallowing movements; TC, teeth-chattering; DIA, diarrhea; WDS, wet dog shakes; EEP, spontaneous erection/ejaculation and penile grooming.
Finally, to test the effects of increased norepinephrine on dopamine in the NAc, we administered idazoxan to a subset of animals and recorded changes in dopamine transient concentrations. Idazoxan did not alter the frequency of dopamine transients, but decreased the average magnitude compared with baseline and vehicle (baseline:73.7±3.6, vehicle: 68.1±5.7, idazoxan: 43.0±1.3 nM, one-way repeated measures ANOVA, F(2,8)=43.13, P=0.0020, Bonferroni post hoc, P<0.01; Supplementary Figure S4), similar to the effects of naloxone-precipitated withdrawal.
DISCUSSION
These findings establish that a single exposure to morphine, followed by precipitated withdrawal, elicits distinct signaling in two forebrain catecholamine circuits. Dopaminergic signaling increased in the NAc during morphine exposure and decreased during withdrawal, but the single exposure did not cause persistent adaptations. Conversely, only precipitated withdrawal elicited norepinephrine release in the vBNST, and this treatment attenuated releasable norepinephrine. Norepinephrine release tracked two withdrawal-associated behaviors and both norepinephrine and withdrawal behaviors were induced without morphine by α2 antagonism. The combination of decreased dopamine output and recruitment of noradrenergic signaling revealed in this work suggests the negative reinforcement model of drug use may develop after the first drug exposure and withdrawal episode.
Drugs of abuse increase dopamine overflow in the NAc (Covey et al, 2014), which helps drive their acutely reinforcing properties, and a recent study showed intravenous morphine administration produces similar efflux (Vander Weele et al, 2014). We gave morphine s.c. since i.v. catheters require rats to be singly housed and social-isolation produces a stress profile and noradrenergic dysregulation similar to morphine-dependent rats (Fox et al, 2015). We found dopamine transients increased in frequency for a much longer duration (>60 min) than in Vander Weele et al (2014), which could arise due to differences in baseline stress or the slower time course of drugs delivered s.c. compared with i.v. This dose of morphine produced immobility in rats, aligning with a previous study showing morphine increased immobility and catalepsy similar to the timing of elevated basal dopamine in the NAc (Sustkova-Fiserova et al, 2014). After drug administration, animals undergo spontaneous withdrawal dependent on the half-life of the drug. To provide a more distinct time point, we precipitated opiate withdrawal with naloxone (Schulteis et al, 1999). Naloxone treatment lowered the frequency of dopamine transients to that of baseline/vehicle; however, the average concentration per transient decreased in the drug-withdrawn group. This effect was not solely due to naloxone, because average transient concentrations only decreased in animals exposed to morphine. Furthermore, in some cases dopamine transients appeared to ‘pause' during somatic withdrawal signs. The decrease in dopamine transients during naloxone agrees with studies showing reduced basal dopamine during withdrawal (Pothos et al, 1991; Weiss et al, 1996), and aligns with previous work showing activation of D2 receptors in the NAc attenuates withdrawal symptoms (Harris and Aston-Jones, 1994). Decreased dopaminergic output supports the allostasis model (Koob and Volkow, 2010) and likely contributes to the withdrawal-induced negative effect.
The role of norepinephrine in drug addiction is often overshadowed by that of dopamine (Weinshenker and Schroeder, 2007), despite evidence that medullary norepinephrine synthesis may be crucial for establishing morphine self-administration (Davis et al, 1975) and conditioned place preference (Olson et al, 2006). The vBNST receives dense noradrenergic projections from the NTS (Forray and Gysling, 2004), and thus is a likely downstream target for norepinephrine's actions in morphine reward. However, we did not detect norepinephrine release in the vBNST during morphine, although elevated norepinephrine in the BNST may develop following chronic drug exposure (Fuentealba et al, 2000). Alternatively, NTS norepinephrine may instead modulate drug-reward through its projections to the NAc (Delfs et al, 1998) where it can regulate dopamine efflux, and presumably the rewarding property of abused drugs through α1 receptors (Mitrano et al, 2012). Norepinephrine signaling is traditionally implicated in the aversive aspects of opiate-withdrawal (Aston-Jones et al, 1999). Indeed, direct infusion of adrenergic antagonists into the BNST suppresses stress-induced reinstatement of drug seeking and conditioned place preference (Leri et al, 2002; Wang et al, 2001), and 3 days of morphine withdrawal produces elevated anxiety and noradrenergic plasticity in the vBNST (McElligott et al, 2013).
In agreement with its role in opiate withdrawal, we found robust norepinephrine release in the vBNST that coincided with somatic withdrawal behaviors. In particular, norepinephrine was linked with SM and the neurons responsible for this reflex are located in the NTS (Kessler and Jean, 1985). Interestingly, releasable norepinephrine concentrations were depleted after withdrawal, reflecting signaling adaptations after the first withdrawal episode. This effect is consistent with previous work showing reduced norepinephrine tissue content in the vBNST after repeated morphine withdrawal (McElligott et al, 2013). We also found norepinephrine release in some vehicle–naloxone-treated animals. We believe this to be a result of removing endogenous opiate tone, as withdrawal signs were also noted in some vehicle–naloxone animals (Figure 4a). The occurrence of release events and withdrawal signs was much greater in animals undergoing withdrawal relative to control, and evoked release was blunted only in animals that underwent precipitated withdrawal. Additionally, when we administered the α2 antagonist idazoxan with naloxone, both norepinephrine overflow and withdrawal behaviors were elicited in the absence of morphine, further supporting norepinephrine's role in withdrawal aversion. Although we found a strong link between norepinephrine and TC/SM, other signaling mechanisms are likely involved in the expression of somatic withdrawal behaviors. For example, blockade of mineralocorticoid receptors markedly attenuates EEP and ptosis (Navarro-Zaragoza et al, 2014), and inhibition of endocannabinoid catabolic enzymes attenuates wet dog shakes (Ramesh et al, 2013). Overall, our data support links between norepinephrine overflow and TC/SM as well as global withdrawal score, and provide mechanistic insight for adrenergic receptor therapy in treating human addicts (Kowalczyk et al, 2015). Moreover, the norepinephrine release during withdrawal uncovered here is the likely origin of adrenergic receptor plasticity in the vBNST (McElligott et al, 2013).
Dopamine and norepinephrine exhibit opposing responses to rewarding and aversive stimuli. Oral infusion of an appetitive tastant produces enhanced dopamine, whereas aversive tastants such as quinine suppress dopamine transients and increase norepinephrine signaling (Park et al, 2012; Roitman et al, 2008). This effect is also seen in animals undergoing reward learning and its extinction (Park et al, 2013), and during presentation of a painful stimulus (Park et al, 2015). Reciprocal catecholamine signaling has interesting implications in the context of the aversive stimulus of drug withdrawal, due to norepinephrine's possible influence on dopaminergic signaling. Glutamatergic inputs from the vBNST exert strong excitatory influence over VTA dopamine neurons (Georges and Aston-Jones, 2002), and norepinephrine's actions through α2A receptors decrease excitatory transmission in the vBNST (Egli et al, 2004). Moreover, during acute opiate withdrawal, norepinephrine can act through β-receptors to increase GABAA inhibition of VTA-projecting BNST neurons (Dumont and Williams, 2004), leading to increased inhibition of the VTA. Activation of β2-adrenergic receptors in the vBNST drives stress-induced reinstatement by releasing CRF in the VTA (Vranjkovic et al, 2014). Although increased VTA CRF can potentiate activity (Ungless et al, 2003), CRF receptor antagonism in the VTA blocks the decrease in NAc dopamine transients caused by aversive quinine infusions (Twining et al, 2015). In this study, we found decreased dopamine concentrations in the NAc during withdrawal. This suppression of dopamine concentrations was further mimicked by elevating norepinephrine with α2 antagonism. Thus, when animals underwent withdrawal, elevated norepinephrine concentrations in the vBNST may have suppressed VTA activity, thereby reducing dopamine transient concentrations in the NAc. At the end of the treatment, the suppression was relieved: regardless of an animal's exposure to withdrawal, direct stimulation of the VTA resulted in equivalent dopamine release, similar to tissue-content findings (McElligott et al, 2013). The reciprocal actions of dopamine and norepinephrine we report during drug exposure and withdrawal may reflect important feedback between catecholamine circuits during rewarding and aversive stimuli.
Overall, the increased dopaminergic signaling we observed during morphine exposure supports a rich literature on dopamine's role in the rewarding properties of drugs. Additionally, the enhanced noradrenergic overflow concurrent with withdrawal signs underscores norepinephrine's importance in mediating opiate-withdrawal aversion. The combination of decreased dopaminergic output and enhanced noradrenergic overflow revealed in this work supports the allostasis model, and suggest that negative reinforcement may emerge after the first exposure/withdrawal episode. Taken together, this real-time view of reciprocal catecholamine signaling provides insight as to how catecholamine circuits in the ventral forebrain may become dysregulated after drug exposure and withdrawal. These adaptations may converge with stress or other risk factors to drive the development of addiction in susceptible individuals, and how they progress longitudinally should be a topic of future investigations.
FUNDING AND DISCLOSURE
This work was supported by NIH grant DA10900 to RMW. The authors declare no conflict of interest.
Acknowledgments
MEF designed the study, collected, and analyzed voltammetric data. NTR assisted with collection and analysis of dopamine transients. MEF wrote the paper and prepared the figures with contributions from NTR and RMW. We thank Dr Elyse Dankoski for comments on an early version of this manuscript.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Aston-Jones G, Delfs JM, Druhan J, Zhu YAN (1999). The bed nucleus of the stria terminalis: a target site for noradrenergic actions in opiate withdrawal. Ann NY Acad Sci 877: 486–498. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE (2000). Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25: 515–532. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ et al (2007). Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci 27: 791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey DP, Roitman MF, Garris PA (2014). Illicit dopamine transients: reconciling actions of abused drugs. Trends Neurosci 37: 200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daberkow DP, Brown HD, Bunner KD, Kraniotis SA, Doellman MA, Ragozzino ME et al (2013). Amphetamine paradoxically augments exocytotic dopamine release and phasic dopamine signals. J Neurosci 33: 452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis WM, Smith SG, Khalsa JH (1975). Noradrenergic role in the self-administration of morphine or amphetamine. Pharmacol Biochem Behav 3: 477–484. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G (2000). Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature 403: 430–434. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones GS (1998). Origin of noradrenergic afferents to the shell subregion of the nucleus accumbens: anterograde and retrograde tract-tracing studies in the rat. Brain Res 806: 127–140. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A (1988). Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85: 5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Williams JT (2004). Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J Neurosci 24: 8198–8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli RE, Kash TL, Choo K, Savchenko V, Matthews RT, Blakely RD et al (2004). Norepinephrine modulates glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropsychopharmacology 30: 657–668. [DOI] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J (2000). Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology 23: 138–150. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW (2005). Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8: 1481–1489. [DOI] [PubMed] [Google Scholar]
- Forray MI, Gysling K (2004). Role of noradrenergic projections to the bed nucleus of the stria terminalis in the regulation of the hypothalamic-pituitary-adrenal axis. Brain Res Brain Res Rev 47: 145–160. [DOI] [PubMed] [Google Scholar]
- Fox ME, Studebaker RI, Swofford NJ, Wightman RM (2015). Stress and drug dependence differentially modulate norepinephrine signaling in animals with varied HPA axis function. Neuropsychopharm 40: 1752–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba JA, Forray MI, Gysling K (2000). Chronic morphine treatment and withdrawal increase extracellular levels of norepinephrine in the rat bed nucleus of the stria terminalis. J Neurochem 75: 741–748. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G (2002). Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci 22: 5173–5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G (1994). Involvement of D2 dopamine receptors in the nucleus accumbens in the opiate withdrawal syndrome. Nature 371: 155–157. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Martin TJ, Co C, Dworkin SI, Smith JE (1995). The effects of intravenous heroin administration on extracellular nucleus accumbens dopamine concentrations as determined by in vivo microdialysis. J Pharmacol Exp Ther 273: 591–598. [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ (2006). Neural mechanisms of addiction: the role of reward-related learning and memory. Ann Rev Neurosci 29: 565–598. [DOI] [PubMed] [Google Scholar]
- Juarez B, Han M-H (2016). Diversity of dopaminergic neural circuits in response to drug exposure. Neuropsychopharm (doi:10.1038/npp.2016.32; e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Kaufling J, Aston-Jones G (2015). Persistent adaptations in afferents to ventral tegmental dopamine neurons after opiate withdrawal. J Neurosci 35: 10290–10303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler JP, Jean A (1985). Identification of the medullary swallowing regions in the rat. Exp Brain Res 57: 256–263. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharm 35: 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk WJ, Phillips KA, Jobes ML, Kennedy AP, Ghitza UE, Agage DA et al (2015). Clonidine maintenance prolongs opioid abstinence and decouples stress from craving in daily life: a randomized controlled trial with ecological momentary assessment. Am J Psychiatry 172: 760–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J (2002). Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci 22: 5713–5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazei-Robison MS, Nestler EJ (2012). Opiate-induced molecular and cellular plasticity of ventral tegmental area and locus coeruleus catecholamine neurons. Cold Spring Harb Perspect Med 2: a012070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott ZA, Fox ME, Walsh PL, Urban DJ, Ferrel MS, Roth BL et al (2013). Noradrenergic synaptic function in the bed nucleus of the stria terminalis varies in animal models of anxiety and addiction. Neuropsychopharmacology 38: 1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrano DA, Schroeder JP, Smith Y, Cortright JJ, Bubula N, Vezina P et al (2012). Alpha-1 adrenergic receptors are localized on presynaptic elements in the nucleus accumbens and regulate mesolimbic dopamine transmission. Neuropsychopharmacology 37: 2161–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Zaragoza J, Laorden ML, Milanes MV (2014). Spironolactone decreases the somatic signs of opiate withdrawal by blocking the mineralocorticoid receptors (MR). Toxicology 326: 36–43. [DOI] [PubMed] [Google Scholar]
- Nestler EJ (2001). Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci 2: 119–128. [DOI] [PubMed] [Google Scholar]
- Olson VG, Heusner CL, Bland RJ, During MJ, Weinshenker D, Palmiter RD (2006). Role of noradrenergic signaling by the nucleus tractus solitarius in mediating opiate reward. Science 311: 1017–1020. [DOI] [PubMed] [Google Scholar]
- Park J, Bucher ES, Budygin EA, Wightman RM (2015). Norepinephrine and dopamine transmission in 2 limbic regions differentially respond to acute noxious stimulation. Pain 156: 318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Bucher ES, Fontillas K, Owesson-White C, Ariansen JL, Carelli RM et al (2013). Opposing catecholamine changes in the bed nucleus of the stria terminalis during intracranial self-stimulation and its extinction. Biol Psychiatry 74: 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Wheeler RA, Fontillas K, Keithley RB, Carelli RM, Wightman RM (2012). Catecholamines in the bed nucleus of the stria terminalis reciprocally respond to reward and aversion. Biol Psychiatry 71: 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM (2003). Subsecond dopamine release promotes cocaine seeking. Nature 422: 614–618. [DOI] [PubMed] [Google Scholar]
- Pothos E, Rada P, Mark GP, Hoebel BG (1991). Dopamine microdialysis in the nucleus accumbens during acute and chronic morphine, naloxone-precipitated withdrawal and clonidine treatment. Brain Res 566: 348–350. [DOI] [PubMed] [Google Scholar]
- Ramesh D, Gamage TF, Vanuytsel T, Owens RA, Abdullah RA, Niphakis MJ et al (2013). Dual inhibition of endocannabinoid catabolic enzymes produces enhanced antiwithdrawal effects in morphine-dependent mice. Neuropsychopharmacology 38: 1039–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeberg NT, Johnson JA, Cameron CM, Saddoris MP, Carelli RM, Wightman RM (2015). Construction of training sets for valid calibration of in vivo cyclic voltammetric data by principal component analysis. Anal Chem 87: 11484–11491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM (2008). Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci 11: 1376–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Heyser CJ, Koob GF (1999). Differential expression of response-disruptive and somatic indices of opiate withdrawal during the initiation and development of opiate dependence. Behav Pharmacol 10: 235–242. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Highfield D, Delfs J, Leung S, Stewart J (2000). Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur J Neurosci 12: 292–302. [DOI] [PubMed] [Google Scholar]
- Sombers LA, Beyene M, Carelli RM, Wightman RM (2009). Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J Neurosci 29: 1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sustkova-Fiserova M, Jerabek P, Havlickova T, Kacer P, Krsiak M (2014). Ghrelin receptor antagonism of morphine-induced accumbens dopamine release and behavioral stimulation in rats. Psychopharmacology (Berl) 231: 2899–2908. [DOI] [PubMed] [Google Scholar]
- Twining RC, Wheeler DS, Ebben AL, Jacobsen AJ, Robble MA, Mantsch JR et al (2015). Aversive stimuli drive drug seeking in a state of low dopamine tone. Biol Psychiatry 77: 895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A (2003). Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron 39: 401–407. [DOI] [PubMed] [Google Scholar]
- Vander Weele CM, Porter-Stransky KA, Mabrouk OS, Lovic V, Singer BF, Kennedy RT et al (2014). Rapid dopamine transmission within the nucleus accumbens: dramatic difference between morphine and oxycodone delivery. Eur J Neurosci 40: 3041–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F (2007). Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol 64: 1575–1579. [DOI] [PubMed] [Google Scholar]
- Vranjkovic O, Gasser PJ, Gerndt CH, Baker DA, Mantsch JR (2014). Stress-induced cocaine seeking requires a beta-2 adrenergic receptor-regulated pathway from the ventral bed nucleus of the stria terminalis that regulates CRF actions in the ventral tegmental area. J Neurosci 34: 12504–12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Cen X, Lu L (2001). Noradrenaline in the bed nucleus of the stria terminalis is critical for stress-induced reactivation of morphine-conditioned place preference in rats. Eur J Pharmacol 432: 153–161. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Schroeder JP (2007). There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology 32: 1433–1451. [DOI] [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE (1996). Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J Neurosci 16: 3474–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.