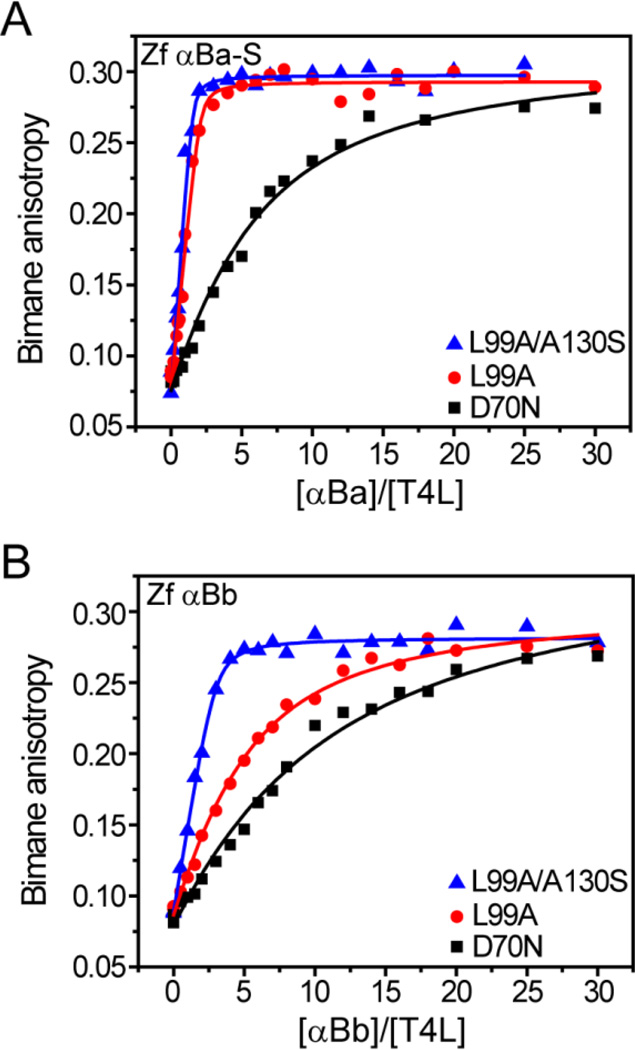
Figure 3.
Zebrafish αB-crystallins demonstrate distinct binding behavior. Similar to αA, the αB paralogs showed increased binding activity toward more destabilized substrates. However, αBa-S (A) binds the T4L mutants with higher affinity and capacity than αBb (B). Binding isotherms were generated in pH 7.2 buffer at 37 °C and the resulting fit parameters are shown in Table 3.