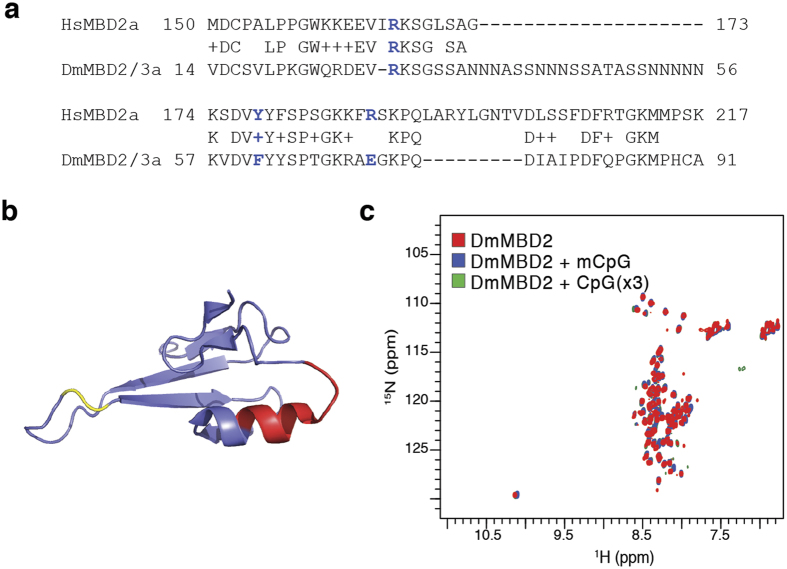
Figure 4. The DmMBD2/3 MBD does not bind DNA.
(a) Alignment of the MBD from DmMBD2/3 with HsMBD2 reveals a large 20 amino acid glutamine rich insertion and a 9 amino acid deletion. In addition, two of the residues critical for binding methylated DNA (highlighted blue) have changed (F61 and E71). (b) A cartoon diagram of the MBD from GgMBD2 demonstrates that the large insertion occurs between residues highlighted in yellow in the loop connecting the central β-strands. The deletion, highlighted in red, eliminates much of the C-terminal α-helix and hydrophobic core of the domain. (c) 2D 15N-HSQC spectra of DmMBD2 MBD in isolation (red) or in the presence of methylated (blue) and unmethylated DNA (green) show characteristic features of an unstructured peptide without any evidence of binding DNA.