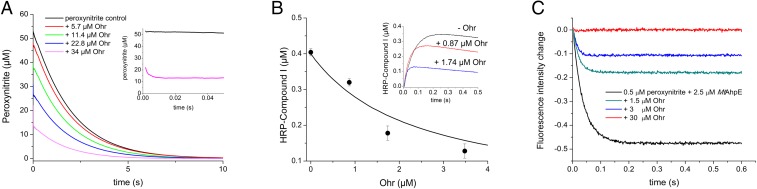
Fig. 5.
Peroxynitrite reduction by XfOhr. Reactions were carried out in potassium phosphate buffer (100 mM), DTPA (100 μM), pH 7.4, at 25 °C. (A) Peroxynitrite (50 μM) was rapidly mixed with reduced Ohr at increasing concentrations, and oxidant decay was followed by its intrinsic absorbance at 310 nm. (B) Peroxynitrite (0.4 μM) was mixed with HRP (10 μM) in the absence and presence of increasing concentrations of reduced Ohr. HRP-compound I formation was monitored at the Soret band. The line represents the expected results simulated using the Gepasi program (76), and considering a simple competition between reduced XfOhr and HRP for peroxynitrite, a rate constant of HRP-mediated peroxynitrite reduction of 2.2 × 106 M−1⋅s−1 and a rate constant of XfOhr-mediated peroxynitrite reduction of 1.2 × 107 M−1⋅s−1, calculated according to Eq. 1. (Inset) Time course of compound I formation by the reaction of HRP (10 μM) with peroxynitrite (0.4 μM) in the absence or presence of increasing concentrations of reduced Ohr. (C) Peroxynitrite (0.5 μM) was mixed with reduced MtAhpE (2.5 μM) in the absence or presence of increasing concentrations of reduced XfOhr, and MtAhpE oxidation was followed by its total intrinsic fluorescence decrease (λ = 295 nm).