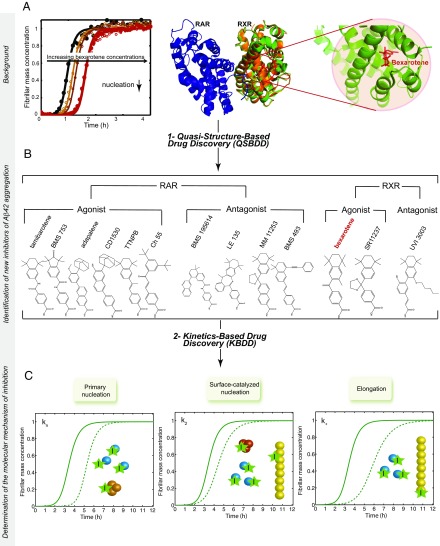
Fig. 1.
Schematic illustration of the drug discovery strategy targeting Aβ42 aggregation described in this work. The strategy consists of two main steps: (i) a QSBDD step and (ii) a kinetics-based drug discovery (KBDD) step. QSBDD consists of identifying potential molecules against Aβ42 aggregation based on the structure of a receptor (here, RXR) of a small molecule (here, bexarotene) shown preferentially to inhibit a microscopic step in Aβ42 aggregation (here, primary nucleation). The rationale behind this strategy is that the instability and transient nature of Aβ42 oligomers make their characterization and, subsequently, the structure-based drug development very challenging. In this study, we considered known agonists and antagonists of RXR based on the possibility that structural similarities could exist between the binding pockets of RXR and Aβ42 oligomers. The strategy was extended to include agonists and antagonists of RAR, given the high structural similarities with RXR. In total, 13 molecules were tested, including bexarotene. (A) This panel is adapted from a study by Habchi et al. (22) and shows the kinetics of 5 μM Aβ42 aggregation (black) in the presence of increasing concentrations of bexarotene (i.e., 1–5 M eq). The structure [Protein Data Bank (PDB) ID code 1XDK] of the RAR receptor (blue) is shown as a dimer with the RXR receptor (orange). The RXR subunit is superimposed onto the subunit of RXR with bexarotene in the binding pocket (green; PDB ID code 4K6I). An enlarged image of the binding pocket is also shown. (B) Structures of all the molecules tested in this study. (C) The KBDD step assesses the effect of a small molecule at the microscopic level. (C) A given molecule can bind monomers, primary oligomers, secondary oligomers, fibrils, or a combination of species, and accordingly affects different microscopic steps that can be characterized quantitatively by global fitting of the aggregation kinetics. The solid lines correspond to the aggregation kinetics of Aβ42 in the absence of an inhibitor. The dotted lines correspond to the kinetics of Aβ42 in the presence of inhibitors that affect a single microscopic step.