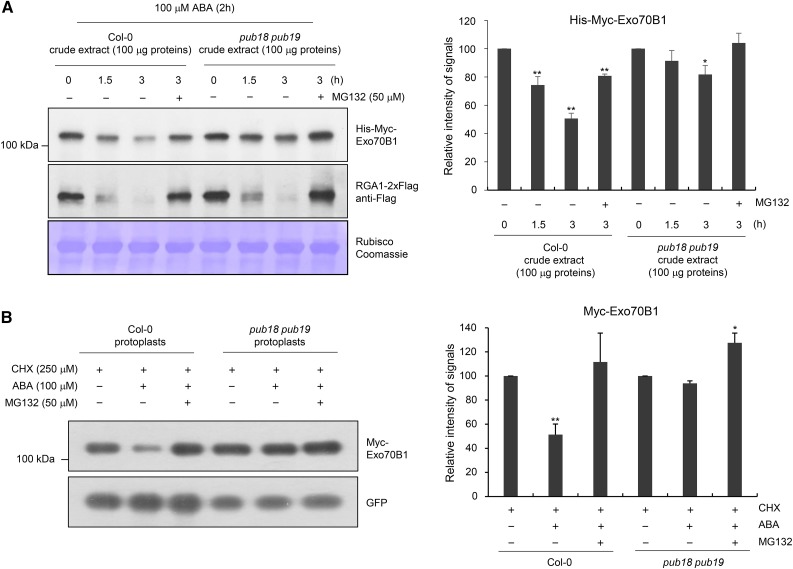
Figure 5.
In Vitro and in Vivo Degradation Assays of Exo70B1.
(A) Cell-free degradation assay of Exo70B1. Bacterially expressed His-Myc-Exo70B1 was incubated with a cell-free crude extract (100 μg total proteins) prepared from ABA (100 μM)-treated mature rosette leaves of wild-type or pub18 pub19 mutant plants in the presence or absence of 50 μM MG132. The time-dependent changes of protein level were monitored by immunoblotting with anti-Myc antibody. The RGA1-2xFlag protein was used as a positive control for the 26S proteasome-dependent degradation. Equal loading of cell-free extracts was confirmed with Rubisco and visualized by Coomassie staining. The protein levels were quantified using ImageJ software. Results are presented as an average value of five independent biological replicates. Error bars represent ±se (n = 5; *P < 0.05, **P < 0.005, one-way ANOVA).
(B) In vivo degradation assay of Exo70B1 in wild-type and pub18 pub19 protoplasts. The 35S:Myc-Exo70B1 and 35S:GFP constructs were transfected into protoplasts freshly isolated from wild-type and pub18 pub19 leaf tissues using PEG-mediated transformation. After overnight transfection, protoplasts were incubated with CHX (250 μM) for 2 h with or without MG132 (50 μM) and ABA (100 μM). Total proteins were extracted and examined by immunoblot analysis with anti-Myc and anti-GFP antibodies. The levels of GFP are shown as transfection efficiency of protoplast and equal loading control. Results are presented as an average value of three independent biological replicates. Error bars represent ±se (n = 6; *P < 0.05, **P < 0.005, one-way ANOVA).