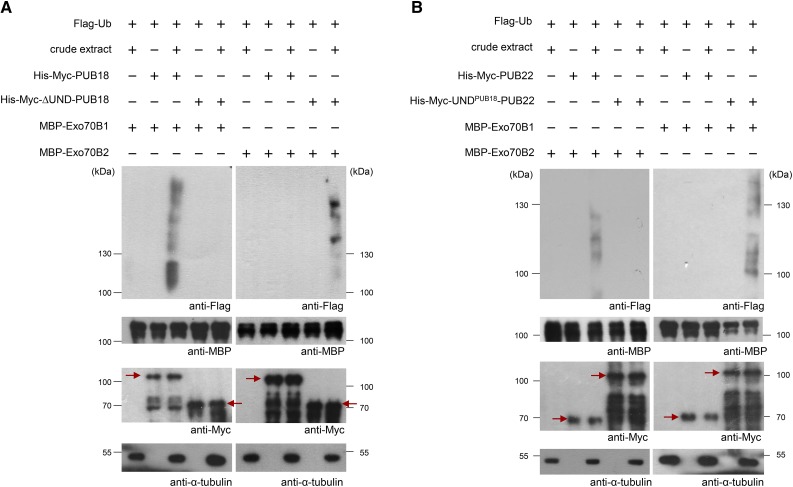
Figure 6.
The UNDPUB18 Domain Is Critical for the Ubiquitination of Exo70B1 and Exo70B2 by PUB18 and PUB22, Respectively.
(A) In vitro ubiquitination assays of Exo70B1 and Exo70B2 by PUB18 and ∆UND-PUB18, respectively. Recombinant His-Myc-PUB18 and His-Myc-∆UND-PUB18 E3 Ub ligases were incubated with MBP-Exo70B1 (left panel) or MBP-Exo70B2 (right panel) with or without crude extract (10 μg total proteins) of ABA (100 μM)-treated pub18 pub19 pub22 pub23 quadruple mutant leaves, along with Flag-tagged Ub (Flag-Ub), E1 (UBA1), MG132 (80 μM), and protease inhibitor cocktail. The reaction mixtures were subjected to immunoblot analysis with anti-Flag, anti-MBP, anti-Myc, and anti-α-tubulin antibodies. Ubiquitinated bands were detected by anti-Flag antibody. Equal loading of each lane was confirmed by anti-MBP and anti-Myc antibodies. The level of α-tubulin was used as a loading control of crude extract. Arrows indicate the migration of the unmodified PUB proteins.
(B) In vitro ubiquitination assays of Exo70B2 and Exo70B1 by PUB22 and UNDPUB18-PUB22, respectively. His-Myc-PUB22 and His-Myc-UNDPUB18-PUB22 E3 Ub ligases were incubated with MBP-Exo70B2 (left panel) or MBP-Exo70B1 (right panel) with or without crude extract (10 μg total proteins) of ABA (100 μM)-treated pub18 pub19 pub22 pub23 quadruple mutant leaves, along with Flag-Ub, E1 (UBA1), MG132 (80 μM), and protease inhibitor cocktail. The samples were resolved by SDS-PAGE and immunoblotted with anti-Flag, anti-MBP, anti-Myc, and anti-α-tubulin antibodies. Ubiquitinated bands were detected by anti-Flag antibody. Equal loading of each lane was confirmed by anti-MBP and anti-Myc antibodies. The level of α-tubulin was used as a loading control of crude extract. Arrows indicate the migration of the unmodified PUB proteins.