Figure 8.
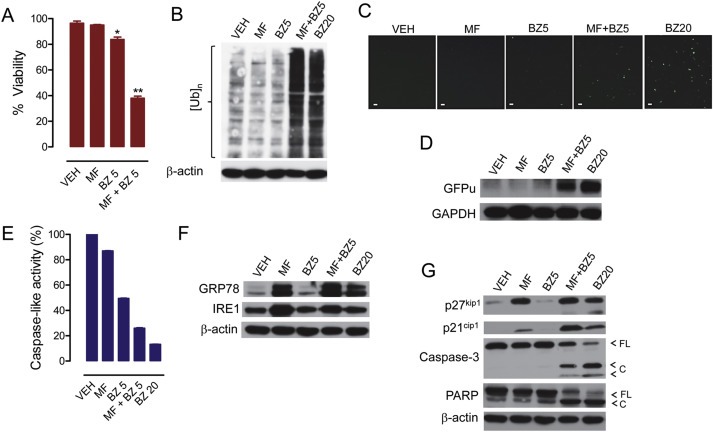
OV2008 cells were exposed for 48 h to 20 μM mifepristone (MF), 5 nM bortezomib (BZ5), 20 nM BZ (BZ20), or the combination of 20 μM MF with 5 nM BZ, and viability was assessed (A). Proteins were isolated, electrophoresed, and subjected to western blot for poly‐ubiquitination (B), ER stress‐related proteins (F), and cyclin dependent kinase inhibitors p21cip1 and p27kip1, caspase‐3, and PARP (G). In a separate experiment, the peptidergic activity of the proteasome was measured in response to similar treatments (E). To further assess the activation of the proteasome in response to cytostatic doses of BZ (5 nM) and MF (20 μM), the cells were transfected with a plasmid expressing GFPu, a green fluorescence protein with carboxyl fusion of an ubiquitination signal sequence (degron CL1); accumulation of GFPu correlates with a reduction in the activity of the proteasome. Panel (C) shows the accumulation of GFPu via fluorescence microscopy (scale bar = 50 μm), whereas panel (D) shows GFPu accumulation by western blot analysis. Results are representative of at least 2 independent experiments with a similar outcome.
