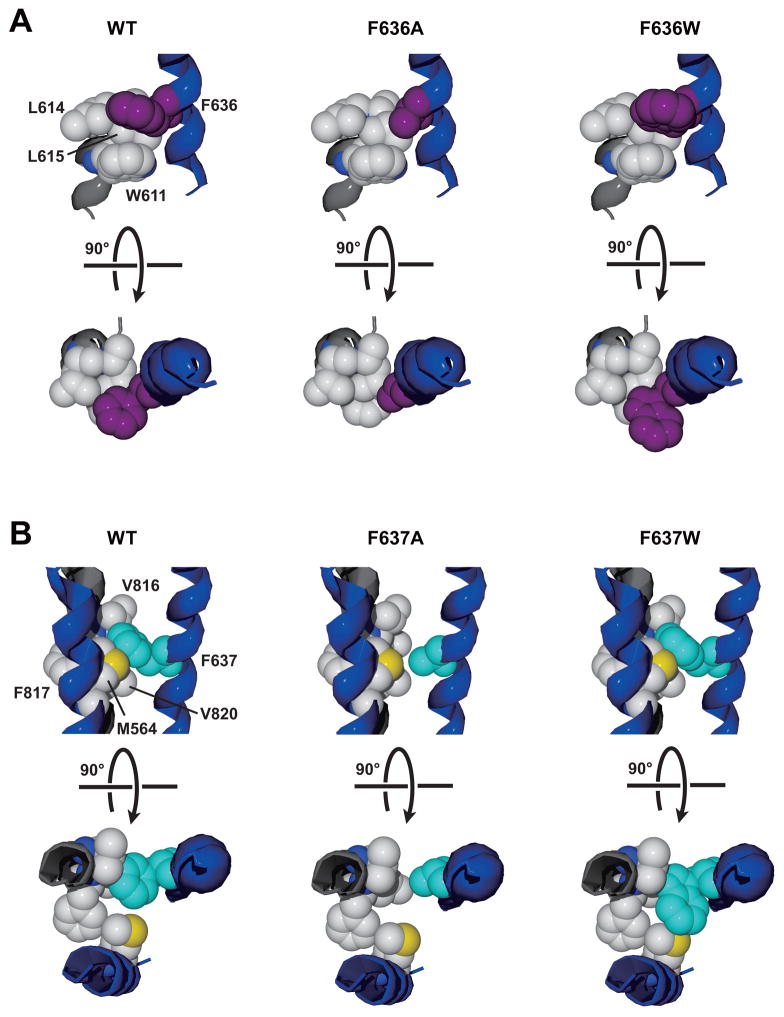
Fig. 9. Altered interactions of phenylalanine side chains at GluN2A positions 636 and 637 with hydrophobic side chains from adjacent helices may explain effects of alanine and tryptophan mutations on mean open time.
A, The environment around the phenylalanine at GluN2A 636 (purple) includes two leucine residues at 614–615 and a tryptophan residue at 611 from the GluN1 M2 domain (left). Introduction of an alanine at 636 (center) may decrease the strength of hydrophobic and aromatic interactions, while introduction of a tryptophan (right) could strengthen these interactions. We propose that weakening the interactions at GluN2A position 636 does not influence open time, but strengthening these interactions slows the closing rate, prolonging open time. B, The environment around the phenylalanine at GluN2A 637 (cyan) includes a phenylalanine at 817 and two valine residues at 816 and 820 from the GluN1 M4 domain, and a methionine at 564 in the GluN2A M1 domain (left). Alanine substitution at 637 may diminish hydrophobic interactions with the GluN1 M4 side chains (center), decreasing mean open time. Tryptophan substitution at 637 (right) may decrease mean open time by introducing a steric clash with the methionine at GluN2A 564.