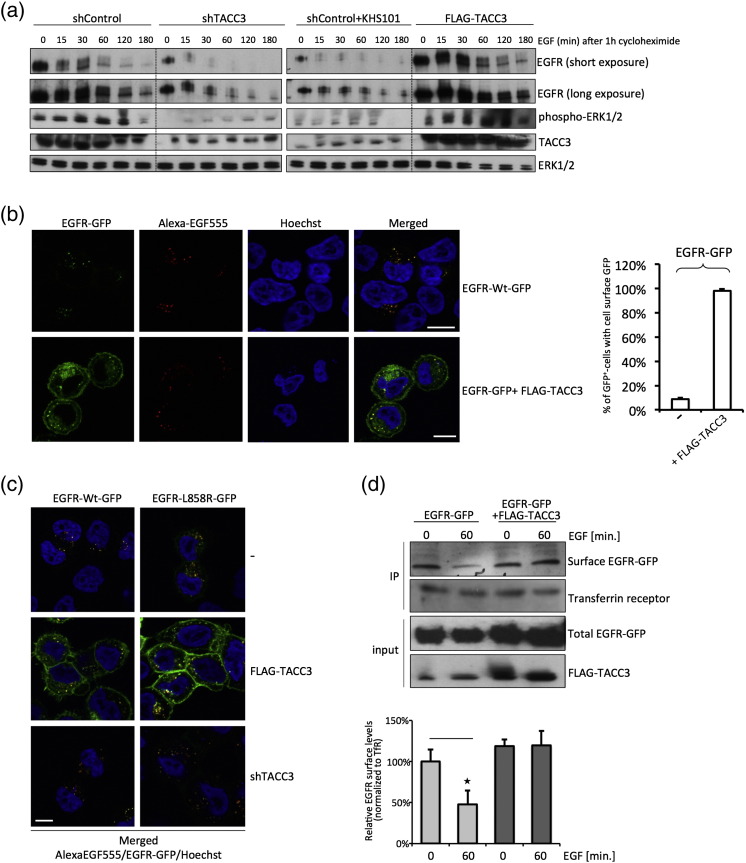
Fig. 4.
TACC3 stabilizes EGFR at the cell surface.
(a) HeLa cells were seeded and transfected with indicated constructs. After 2 days, cells were starved overnight, pre-treated with KHS101 (5 μM) for 3 h (where indicated), treated with cycloheximide for 1 h, and stimulated with EGF (100 ng/μl) for indicated times to assess EGFR stability. Cells were then lysed and subjected to Western blot analysis using EGFR, phospho-ERK1/2, ERK1/2, and TACC3 antibodies.
(b) HeLa cells stably expressing FLAG-TACC3 and control HeLa cells were seeded onto glass coverslips and transfected with EGFR-GFP. Then, 2 days after transfection, cells were starved overnight, then stimulated with EGF (100 ng/μl) for 60 min, and fixed;fluorescence microscopy was performed. Scale bar represents 10 μm. Then, 30 cells were counted in two independent experiments, and overall cell surface GFP was assessed.
(c) HeLa cells were seeded onto glass coverslips and transfected with EGFR-GFP or EGFR-L858R-GFP and FLAG-tagged TACC3. Then, 2 days after transfection, cells were starved overnight, then stimulated with Alexa-EGF555 (100 ng/μl) for 60 min, and fixed; fluorescence microscopy was performed. Scale bar represents 10 μm.
(d) HeLa cells were transfected with FLAG-tagged TACC3 or empty FLAG-vector control. Then, 2 days after transfection, cells were starved overnight and stimulated with EGF (100 ng/μl) for 60 min. Cell surface proteins were biotinylated and immunoprecipitated using streptavidin-coupled beads. Cell surface EGFR levels were assessed by Western blot analysis of immunoprecipitated samples using EGFR antibody and transferrin receptor antibody. EGFR and TACC3 expression levels of input samples were assessed. The densities of the EGFR bands were quantified using ImageJ software and normalized against transferrin receptor. Data are presented as means ± standard deviation. Asterisks show p-values: ⁎p < 0.05.