Figure 6.
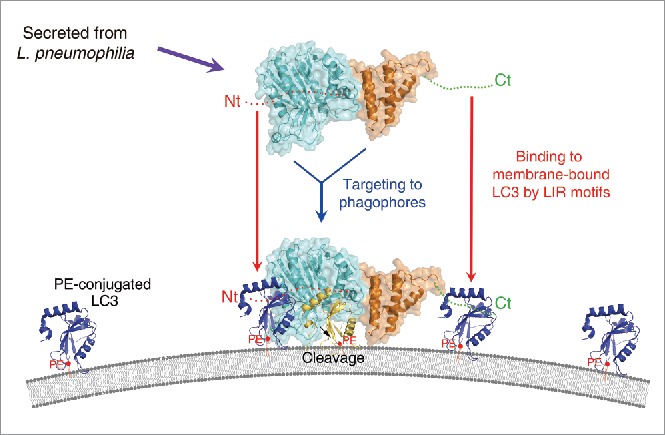
A plausible model for the mode of action of RavZ. The RavZ protein is secreted from L. pneumophila and then targeted to the phagophore or autophagosomal membrane. The catalytic domain of RavZ possessing cysteine protease activity is colored cyan and the membrane-targeting domain possessing binding affinity for the PtdIns3P-rich membrane23 is colored orange. The LIR motif containing the N- and C-terminal regions are indicated as red and green dots, and labeled as Nt and Ct, respectively. PE-conjugated and membrane-anchored LC3 is shown as a blue ribbon and PE is shown as a red ball with tails. Cleaved LC3 (by RavZ) is shown in yellow. RavZ possesses higher binding affinity for LC3 than ATG4B because it has 2 independent LIR motifs at the N- and C-terminal regions (Nt and Ct, respectively). We propose that RavZ achieves greater binding affinity for membrane-bound LC3 molecules (blue ribbon) by using the N- and C-terminal LIR motifs, and this event is critical for correct orientation to facilitate cleavage of the LC3–PE substrate generating a product that cannot be reconjugated to PE (yellow ribbon). Using this elegant and superior mechanism with RavZ as the key player, L. pneumophila has evolved an effective survival mechanism against host cell autophagy.
