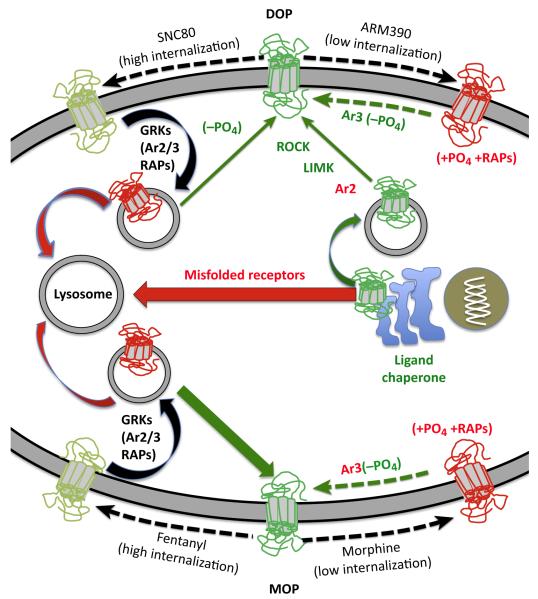
Figure 1. Differences in Opioid Receptor Regulation by Low- and High-Internalizing Agonists.
The cartoon depicts the DOP (δ-opioid receptor) and MOP (μ-opioid receptor) (receptors in red have lost membrane signaling ability) after activation by a high-internalizing agonist; fentanyl (for MOP), or SNC80 (for DOP), and a low-internalizing agonist, morphine (for MOP), or ARM390 (for DOP). Red arrows represent processes that downregulate opioid receptors (lysosomal degradation of agonist-internalized receptors or lysosome-targeted misfolded receptors following de novo synthesis). Green arrows represent mechanisms of resensitization or enhancement of receptor activity. Several membrane-permeable DOP agonists and antagonists act as chaperones for DOP resulting in increased levels of surface receptors [108]. Externalization of vesicle-stored receptors can be enabled by a ROCK/LIMK mechanism, which is held in check by arrestins (Ar). High-internalizing agonists of DOP and MOP recruit G protein receptor kinases (GRKs), arrestins 2 or 3, and other receptor-associated proteins or RAPs (Ras-related proteins) (left side of the figure) to promote internalization as well as arrestin-dependent and vesicle-mediated signaling [109]. Once internalized the receptors are targeted either to lysosomes or recycled back to the cell surface in a resensitized, dephosphorylated (−PO4) state. MOPs effectively recycle back to the membrane (robust green arrow) whereas DOPs are predominantly targeted to lysosomes for degradation (robust red arrow)[110] although, unlike many other receptors, this appears not to require receptor ubiquitination [111]. By contrast, activation by low-internalizing agonists of MOP and DOP only weakly recruits arrestin 3, and desensitization is reported to be dependent upon other kinases such as JNK and PKC (+PO4). Resensitization of DOP and MOPs, presumably by dephosphorylation, following low-internalizing agonist desensitization is differentially regulated by arrestin 3. In the case of MOP, arrestin 3 (Ar3 in red) attenuates resensitization [112] whereas for DOP arrestin 3 (Ar3 in green) facilitates resensitization [19].