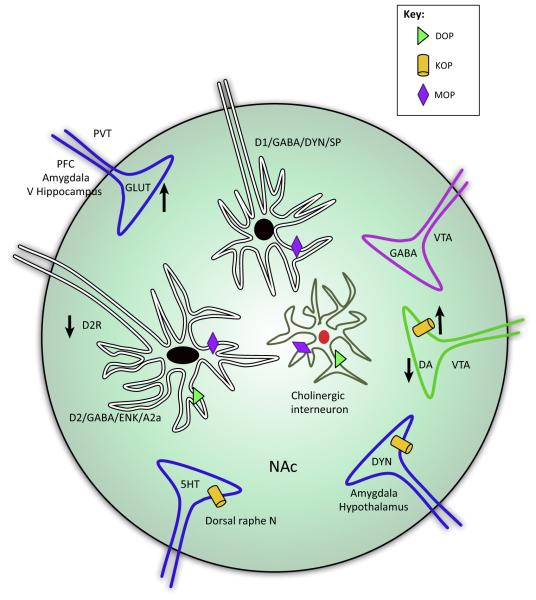
Figure 2. Schematic Cartoon of How Inputs to the Nucleus Accumbens (NAc) Are Modified by Chronic Opioids and their Contribution to Negative Affect.
This cartoon depicts sites of neuronal allostasis in the NAc, potentially contributing to negative affect following chronic opioid treatments. Dopamine neurons from the ventral tegmental area (VTA) project onto two types of GABAergic medium spiny neurons (MSNs) defined by the ability of dopamine to inhibit or excite these neurons within the NAc. Excitatory MSNs are characterized by expression of dopamine D1 receptors, GABA, dynorphin, and substance P, whereas inhibitory MSNs are characterized by the expression of dopamine D2, GABA, enkephalin, and adenosine A2a receptors. The NAc also receives excitatory (glutamatergic) input from the basolateral amygdala, ventral hippocampus, and prefrontal cortex that drives drug reinforcement, whereas input from the paraventricular nucleus of the thalamus can drive reward or aversion associated with withdrawal [78,101]. Chronic morphine is known to increase or decrease long-term potentiation (LTP) and long-term depression (LTD) of some of these glutamatergic inputs, and this may depend on the method of morphine administration (contingent versus non-contingent) [75]. Other inputs originate from the dorsal raphe nucleus, hypothalamus, and GABAergic projection neurons from the VTA. κ-Opioid receptors (KOPs) are expressed on the axon terminals of dopamine neurons, where they can inhibit dopamine release. Chronic opioid treatment increases the expression of KOPs and likely contributes to dopamine hypofunction in this circuitry. Dynorphin, the endogenous agonist at KOP, is released by excitatory MSNs; however, projection neurons from the amygdala and hypothalamus are also sources of striatal dynorphin. KOPs are also present on the axon terminals of these dynorphin projection neurons. δ-Opioid receptors (DOPs) and μ-opioid receptors (MOPs) are expressed on cholinergic interneurons and are involved in modulating reward and motivation, but it is unknown to what extent they contribute to opioid tolerance and opponent processes. MOPs are also present on both excitatory, D1, and inhibitory, D2, MSNs, and activation of these receptors on excitatory D1 neurons is sufficient to drive reward-like behavior [39]. By contrast, DOPs appear to be expressed in D2-, but not D1-, enriched MSNs [114]. Synaptic insertion of GluA2-lacking AMPA receptors in D2 MSNs is implicated in mediating the aversion as a result of activation of the paraventricular-accumbens circuitry during withdrawal [78]. Abbreviations: α2a, A2a cholinergic receptor; DA, dopamine; D1/2R, dopamine receptor 1/2; DYN, dynorphin; ENK, enkephalin; GLUT, glutamate; 5HT, serotonin; PFC, prefrontal cortex; PVT, paraventricular thalamic nucleus; SP, substance P; V Hippocampus, ventral hippocampus.