Fig 5. The HZA enhancer directly bound the HIZR-1 DNA-binding domain.
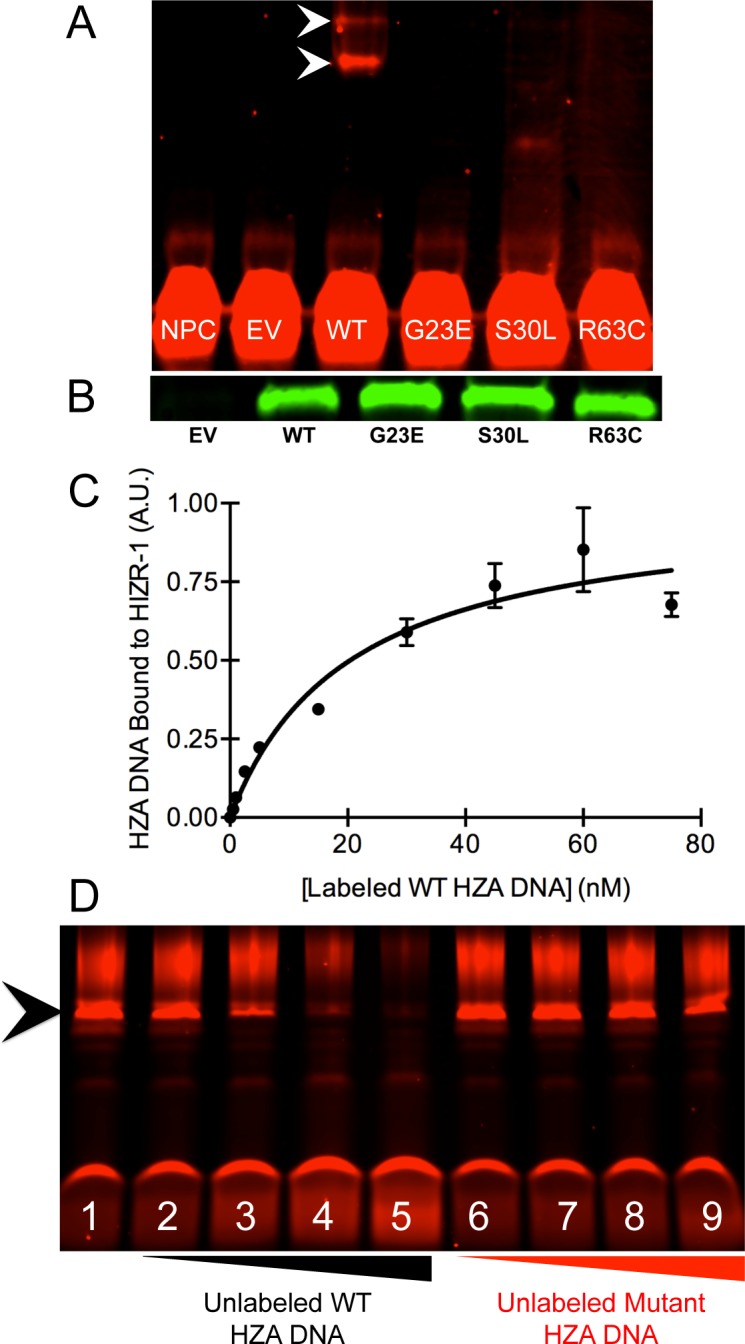
(A) Infrared image of an electrophoretic mobility shift assay (EMSA). Labeled HZA enhancer DNA bound partially purified, full-length, wild-type (WT) HIZR-1 protein (arrowheads) but not mutant (G23E, S30L, or R63C) proteins. NPC, no protein control: EV, empty vector. The S30L lane contains a faint, rapidly migrating band of unknown significance. (B) Equivalent WT and mutant HIZR-1 protein amounts demonstrated by western blotting. (C) A constant amount of partially purified, full-length, wild-type HIZR-1 protein was incubated with variable concentrations of labeled, wild-type HZA DNA. Values are the average amount of labeled HZA DNA that displayed retarded migration +/- S.D. determined by image analysis and expressed in arbitrary units (A.U.). At least two technical replicates were performed for each HZA DNA concentration. Retarded migration indicates the DNA is bound to HIZR-1 protein. Nonlinear regression was used to calculate a dissociation constant of 20.4 +/- 6.8 nM. (D) A constant amount of partially purified, full-length, wild-type HIZR-1 protein was incubated with a constant amount of labeled wild-type HZA enhancer DNA. The interaction between the labeled DNA and HIZR-1 protein was competed with no unlabeled DNA (Lane 1) or increasing concentrations (5, 50, 500, or 5,000 nM) of unlabeled wild-type HZA DNA (Lanes 2–5) and unlabeled mutant HZA DNA (Lanes 6–9). The mutant HZA DNA had the identical flanking sequences but the order of the 15 base pair HZA was randomized. Lanes 3–5 display competition of binding, as evidenced by diminishment of the intensity of the retarded band (arrowhead). By contrast, lanes 7–9 do not display diminishment of the intensity of the retarded band, indicating that the mutant HZA DNA does not compete for binding to the HIZR-1 protein.
