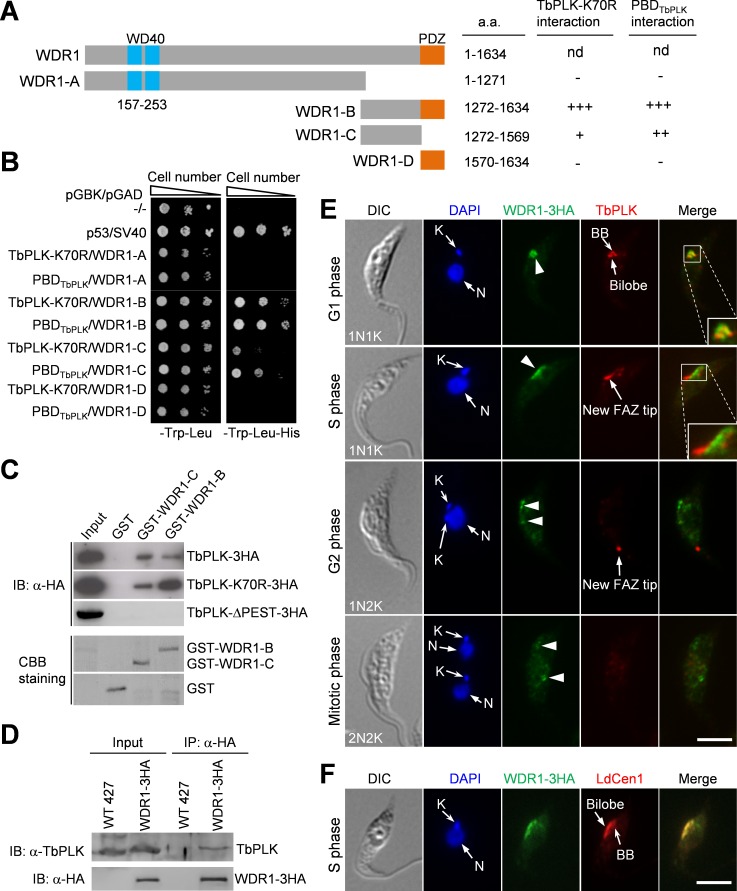
Fig 2. WDR1 interacts with TbPLK through its C-terminal domain and partially overlaps with TbPLK in the basal body and the bilobe at G1 phase.
(A). Schematic drawing of the domains in WDR1 and the truncation mutants of WDR1, and summary of yeast two-hybrid results. nd, not done because we failed to clone the full-length WDR1 gene into the yeast expression vectors despite multiple attempts. (B). Directional yeast two-hybrid assay to detect the interaction between TbPLK-K70R, PBDTbPLK and WDR1 truncation mutants. (C). WDR1, through its C-terminal domain, interacts with the PEST motif of TbPLK in vitro. The truncation fragments of WDR1 were expressed as GST-fusion proteins in E. coli, purified and used to pull down TbPLK-3HA, TbPLK-K70R-3HA, and TbPLK-ΔPEST-3HA from T. brucei cell lysate. CBB, coomassie brilliant blue. (D). WDR1 interacts with TbPLK in vivo in T. brucei, as demonstrated by co-immunoprecipitation. Endogenously 3HA-tagged WDR1 was immunoprecipitated with anti-HA antibody conjugated to protein G sepharose beads, and immunoprecipitated proteins were immunoblotted with anti-TbPLK antibody and anti-HA antibody to detect TbPLK and WDR1-3HA, respectively. (E). Subcellular localization of WDR1 and TbPLK during the cell cycle. Cells expressing endogenously 3HA-tagged WDR1 were co-immunostained with FITC-conjugated anti-HA mAb and anti-TbPLK pAb, and counterstained with DAPI for nuclear (N) and kinetoplast (K) DNA. Scale bar: 5 μm. (F). Localization of WDR1 to the basal body and the bilobe region during the S phase of the cell cycle. Cells were co-immunostained with FITC-conjugated anti-HA mAb to label WDR1-3HA and anti-LdCen1 pAb to label the basal body and the bilobe. Among the 115 S-phase cells examined, all of them showed WDR1-3HA localization to the basal body and the bilobe region. Scale bar: 5 μm.