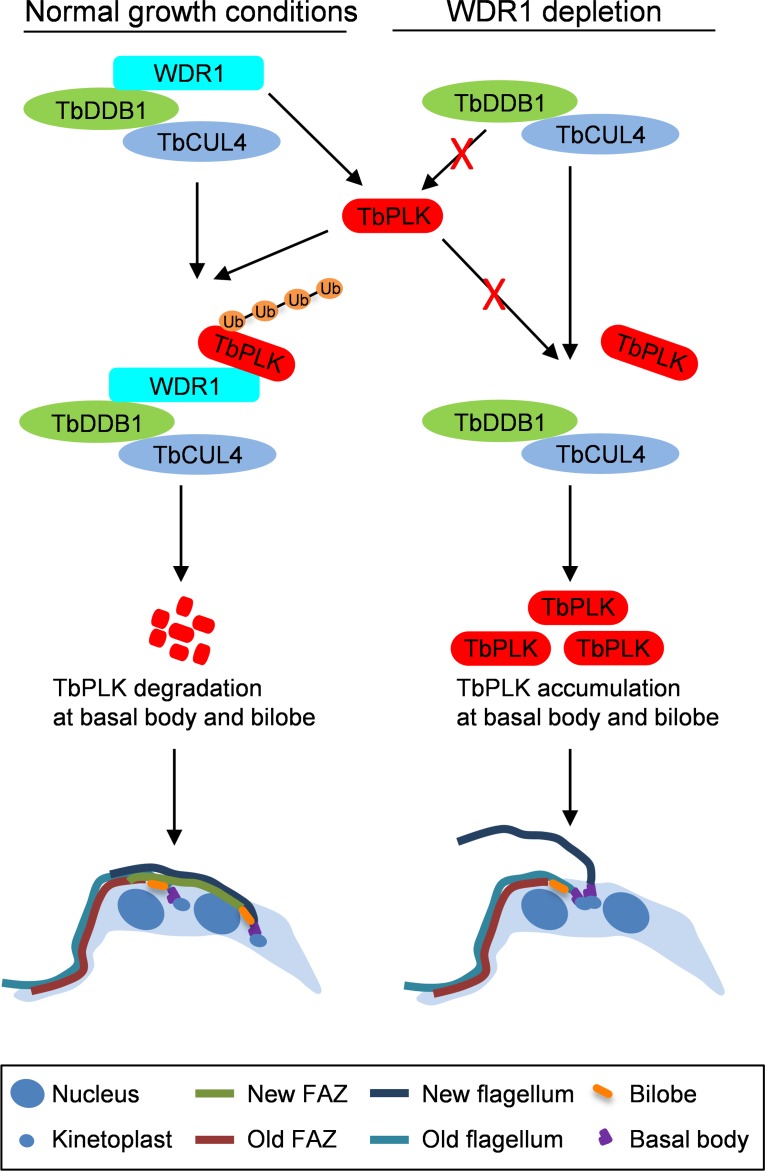
Fig 8. Model of the role of CRL4WDR1 in controlling TbPLK protein abundance to promote bilobe duplication, basal body segregation and FAZ assembly.
WDR1 in the CRL4 ubiquitin ligase complex recognizes the PEST motif in TbPLK. Binding of TbPLK to WDR1 causes TbPLK ubiquitination by CRL4WDR1 and subsequent degradation of TbPLK in the basal body and the bilobe after the G1/S cell cycle transition. This degradation of TbPLK in the basal body and the bilobe promotes bilobe duplication, basal body segregation, FAZ filament assembly, flagellum attachment and faithful cytokinesis. When WDR1 is depleted, TbPLK is not recruited to the TbCUL4-TbDDB1 complex for ubiquitination, leading to TbPLK accumulation in the basal body and the bilobe, where it may continuously phosphorylate the bilobe protein TbCentrin2. This accumulation of TbPLK inhibits bilobe duplication, impairs basal body segregation, disrupts the assembly of the new FAZ filament, which causes flagellum detachment, and blocks cytokinesis.