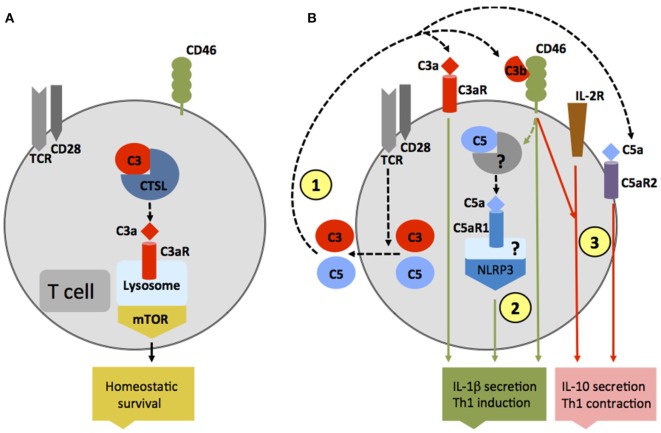
Figure 2.
Autocrine and intracellular complement activation. (A) In resting T cells, C3 is processed intracellularly by the protease cathepsin L to generate bioactive C3a that sustains low-level mechanistic target of rapamycin (mTOR) activity via the engagement of the intracellular C3aR expressed on lysosomes and thus contributes to homeostatic survival of CD4+ T cells. (B) Local complement activation is triggered when activating signals [here, T cell receptor stimulation or toll-like receptors activation on antigen-presenting cells (not shown)] initiate secretion of preformed C3, C5, factor B, and factor D located in cellular storages, leading to C3 and C5 convertase formation in the extracellular space as well as on the cell surface—and the generation of C3a, C3b, C5b, and C5a (1). These complement fragments bind to their respective receptors on the T cell surface and induce Th1 induction with interferon (IFN)-γ secretion. C5 is also processed intracellularly by a yet unknown protease/convertase into C5a and C5b, and this process is increased through CD46-mediated signals. Intracellular C5a engages the intracellular C5aR1, which triggers NLRP3 inflammasome assembly and intrinsic IL-1β secretion that sustains Th1 induction in an autocrine fashion (2). Importantly, autocrine CD46 activation in conjunction with IL-2R signaling also induces IL-10 co-production in Th1 cells and the transition into a (self)regulative Th1 contraction phase (3). This “IL-10 switch” is accompanied by autocrine surface engagement of the C5aR2 via surface-shuttled C5a/C5a-desArg (3) through an as yet undefined signaling pathway (but possibly through direct suppression of intracellular C5aR1 activity, not shown).