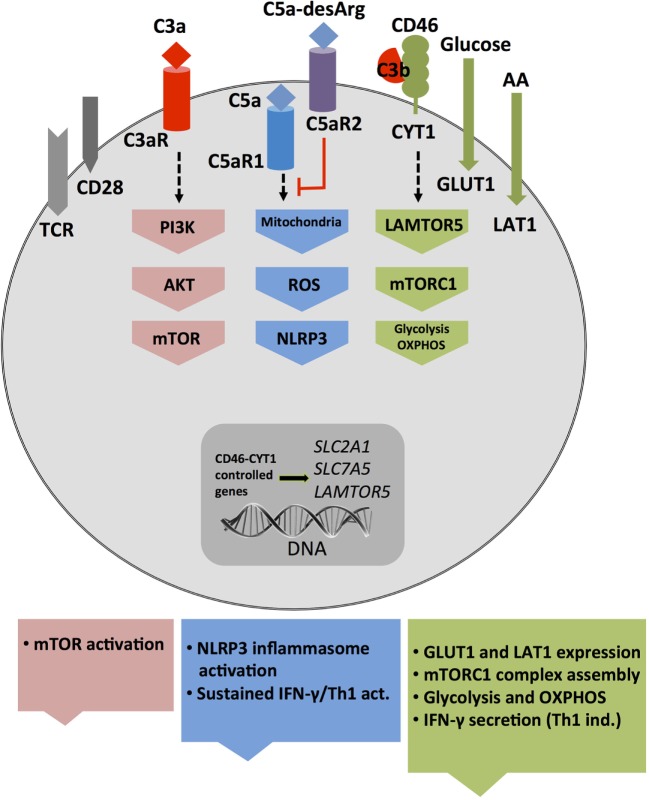
Figure 4.
Autocrine and intracellular complement drives key T cell metabolic pathways during T helper cell type 1 induction. T cell receptor and CD28-driven autocrine engagement of CD46 CYT-1 (by C3b) leads to upregulation of genes coding for the glucose transporter GLUT (SLC2A1), and the amino acid channel LAT1 (SLC7A5), allowing for increased influx of glucose and amino acids (AA) into the cell (2). In parallel, CD46 CYT-1-mediated signals induce increased expression of LAMTOR5, and via this assembly of the lysosome-based machinery enabling AA sensing via mTOR complex 1 (mTORC1), which then leads the induction of glycolysis and OXPHOS required for interferon (IFN)-γ production. Surface activation of the C3aR by C3a activates PI3K and AKT and further sustains mechanistic target of rapamycin (mTOR) activity. Intracellular C5aR1 stimulation (at a not yet defined cellular compartment) drives mitochondrial reactive oxygen species (ROS) production, which induces NLRP3 inflammasome activation and maintenance of IFN-γ secretion during T cell migration into the (inflamed) tissues.