Abstract
Purpose: Lipid oxidation and rheological properties are the main qualitative parameters determined in food emulsions. Salad dressings are food emulsions important in our daily diet, but conventional salad dressings have high amounts of cholesterol and saturated fatty acids because of egg yolk in their formulations. There are many studies on the modification of salad dressing formulations to replace egg yolk and saturated fats. The present study describes new formulation of salad dressing with olive oil and apple vinegar to produce a functional food product.
Methods: This study investigated the qualitative properties, oxidative stability, rheological behavior and microstructure of the salad dressing without egg yolk. Oil-in-water emulsions were prepared with virgin olive oil and apple vinegar stabilized with various percentages of xanthan (T1: 0.25%, T2: 0.5%. T3: 0.75%). Samples were stored at refrigerator for 90 days and experiments were performed at production day and during storage.
Results: The obtained results showed that peroxide value was increased for all samples during storage, but it was at an acceptable level. Fatty acid changes were not significant during storage. Droplet size was reduced by increasing xanthan gum. T2 had the best rheological properties during storage. Generally, T2 and T3 had higher scores and were more acceptable in organoleptic assay.
Conclusion: Obtained results showed that T2 had suitable qualitative and rheological properties and can be a proper egg yolk free salad dressing to introduce to the market.
Keywords: Lipid oxidation, Xanthan gum, Rheological behavior, Emulsion stability, Microstructure
Introduction
Salad, usually served as a side dish or a full food, is a very fashionable food choice. Sauces are added to salads and other foods to improve or alter their flavor and make them more palatable.1
Olive oil is a part of the Meditrainian diet, and it has positive roles in the prevention of some illnesses. It has high oxidative stability because it is rich in monounsaturated fatty acids, antioxidants and phenolic compounds.2 In addition to having all these benefits, the popular taste of olive oil makes it the best candidate to be selected as oil base in the salad dressings.3
Apple vinegar has many bioactive compounds and has been shown to have many positive effects on health. This ingredient also decreases pH; therefore, it increases products shelf life and retard spoilage.4
Salad dressings are emulsions which need to have emulsifiers to be stable. Generally, egg yolk is used as an emulsifier in their production. Egg yolk has high cholesterol and saturated fatty acid content, which may promote cardiovascular diseases. Also, egg yolk can increase the risk of microbial contamination and reduce shelf life of products. For this reason, there is more interest to introduce new emulsifiers as alternatives to egg yolk. Some suitable options are hydrocolloids and polysaccharides.5,6
Xanthan gum is one of the widely used hydrocolloids in food industry which is an anionic heteropolysaccharide made by the “Xanthomonascampestris”.7 Xanthan solutions are highly semiplastic and show high viscosity, so they are used in oil-in-water emulsions so as to develop aqueous phase viscosity as well as to prevent them from creaming and phase separation.8,9
Lipid oxidation is a predominant reason for quality deterioration of oils, fats and foods with high content lipids during storage. The interaction between unsaturated lipids and oxygen-active compounds is the basis for this reaction. Different factors can affect oxidative stability of oil-in-water emulsions, including lipid types, oxygen concentrations, antioxidants, interfacial characteristics, droplet specification, interactions with aqueous phase components and the presence of prooxidants.10 The role of polysaccharides in lipid oxidation in o/w emulsions system has been shown in some studies.11 The polysaccharide increases aqueous phase’s viscosity and thus prevents oxygen dispersion and reduces the oil droplet's motion, reducing their collision possibility. Also, it chalets the metal ion and results in high oxidative stability.12
In this study, to produce healthy and nutritious salad dressings, xanthane was used to replace egg yolk. The purpose was studying the effect of xanthan gum on oxidative stability, physicochemical, rheological characteristics, microstructure and droplet size distribution of olive oil-in-water and apple cider vinegar emulsions.
Materials and Methods
Materials
Xanthan gum was purchased from Sigma Chemical Co. (USA). Virgin olive oil was produced by Iranian zeytoonak company (Alma). Apple vinegar was produced by the traditional method. Other ingredients included sugar, salt, garlic powder and mustard, which were purchased from local market. All chemicals were of reagent grade and were bought from Merck KGaA 64271 Darmstadt Germany.
Preparation of salad dressing
The researchers prepared oil-in-water emulsions (45% w/w) based on the method described by A. Paraskevopoulos et al as follows:13 half the olive oil was mixed with xanthan gum by stirring with a glass stirrer. Then all of the water, solid materials, apple vinegar and the remaining oil were mixed together in the mixer (Richmond) at the speed of 9500 rpm at room temperature for 3 minutes. Then, the mixture of oil and gum was added to the mixer.The emulsions were prepared by stirring for 1 minute in the same conditions. Xanthan gum concentration was 0.25% (T1), 0.5% (T2), 0.75% (T3).
All samples were kept at 4°C for 90 days, and PV were tested on the first day and every 30 days, but rheological properties and fatty acid determination and organoleptic test were determined on the first day and each 90 days. Microstructure was evaluated on the first day of production.
Peroxide value
Peroxide value (PV) was determined by method (AOAC, 2000, Method Number 965.33b) on the oil extracted from salad dressing (by Jacoben et al method) during 90 days storage in intervals of 30 days.14
Fatty acid profile
Transformation of oil to fatty acid methyl ester was done by European official methods of Analysis (AOCS Ce 2-66(1997)). The profile of fatty acid was determined by gas chromatograph (GC-1000, DANI, Italy) with flame ionization detector in accordance with the method explained by Azadmard-Damirchi et al.15
Rheological measurements
The samples, steady and oscillatory shear rheological properties were measured through the use of a shear-rate ⁄ shear stress controlled Rheometer (Anton Paar, MCR301, Austria) with concentric cylinder system (CC27: Ti, ø cup [mm]: 28.92, ø bob [mm]: 26.66) at 25°C ± 0.01). We measured shear stress as a function of shear rate over a wide range from 0 to 100 s-1. To clarify the influence of different amounts of xanthan gum on the rheological behavior of emulsions, their shear stress vs. shear rate data were fitted with some reported mathematical models including;Power law (Eq. 1), Bingham (Eq. 2), Herschel-Bulkley (Eq. 3) and Casson (Eq. 4).
| (1) |
| (2) |
| (3) |
| (4) |
[σ represents shear stress (Pa); κ, consistency coefficient (Pa.s)n; γ , shear rate (s-1); µpl, plastic viscosity (Pa.s); σ0, yield stress (Pa); κ1, Casson plastic viscosity (Pa.s); n, flow behavior index].
R2 was used to evaluate the fitting of experimental data with models.16
Determination of the viscoelastic properties
Dynamic oscillatory test was performed over an angular frequency (ω) in the range of 0.05–100 rad/s at constant strain. The obtained mechanical spectra were:
G′: storage modulus
G″: loss modulus
tan δ= G″/ G′: loss tangent components
η* = G*/ ω: complex viscosity
Then, the curves of G′, G″, η* and tan δ versus frequency were obtained, as described by Steffe JF (1996).17 The Bohlin’s parameters were determined from the equation: G* = Aω1/z, where: z represents coordination number (dimen-sionless) and A represents proportional coefficient (Pa s1/z).18,19 The measurements were obtained by doing three replicate experiments for each sample. Rheological parameters were specified on the first day of production and after 3 months storage.
Microstructure
The droplets of emulsions were observed with a light microscope (CETI,UK) according to the method described by S. Pei Ng et al. Images of the samples microstructures were taken by a digital camera (Sony, Zeiss, Japan) jointed to the microscope at a magnification of ×20.20
Droplet size distribution
Measuring the particle size distribution of the salad dressing samples was conducted by laser light scattering device (Cilas 1090 particle size analyzer (Orleans) and by method of Karimi et al.20 Results were reported by Particle sizer device software and according to Fran Hofer model as the following parameters:
D[4,3] =Σnidi4 / Σnidi3 (6)
Span = Dv90 − Dv10 /Dv50 (7)
where Dv10, Dv50 and Dv90 represent 10%, 50% and 90% of the droplets diameters in all the emulsion volumes with diameters smaller or equal to these values.
ni is the number of droplets of diameter di.20
Organoleptic evaluation
Sensory analysis was performed by quantitative descriptive test through a general panel consisting of 15 assessors. The features were assessed for color, flavor, texture and acceptance in terms of customer-friendliness and compared to similar products on the market. The ranking was as follows: (1: very weak, 2: weak, 3: average, 4:favorable, 5:very well). No information was given to the panelists and all of the samples were coded randomly.
Statistical analysis
All measurements were made in triplicate on samples. The data were analyzed using SPSS (version 20) and results were stated as mean and standard error of mean. One-way analysis of variance (ANOVA) and LSD test were the data analysis tools used to establish the significance of the differences among the mean values at the 0.05 significance level. Repeated measure test was employed to express the significant difference between the mean values obtained in the course of time. MATLAB software (v. R2012a) was used for the rheological models.
Results and Discussion
Oxidative stability
Peroxide value
Lipid oxidation occurs on the surface layer of the oil droplets in oil-in-water emulsions. The interaction between lipid hydroperoxides at the droplet surface and transition metals, from the aqueous phase, is the popular reason for oxidative inconsistency which has been suggested by many studies.21
PV range for olive oil is 15 (meqO2/kg oil); therefore, all of the salad dressings PVs were acceptable. The sample with 0.5% of xanthan had a significantly lower PV (P<0.05) than the other samples. PV was increased in all samples and reached from 11.2 to maximum level of 12.88 during storage (Table 1).
Table 1. Peroxied value (meq O2/kg oil ) of olive oil– apple cider vinegar salad dressings during storage at 4oC .
| Samples | Storage (days) | |||
| 1 | 30 | 60 | 90 | |
| T1 | 11.500±0.086baC | 11.920±0.083aB | 12.443±0.120aA | 12.710±0.092aA |
| T2 | 11.240±0.090bC | 11.503±0.077bB | 12.076±0.086bABC | 12.670±0.055aA |
| T3 | 11.766±0.150aB | 11.956±0.067aB | 12.426±0.083aA | 12.88±0.112aAB |
Mean ± Standard errors. Means within the same column (a, b, c) and the same row (A, B, C, D) with different letters are significantly different (p < 0.05).
Level of xanthan in samples: T1: 0.25%, T2: 0.5% and T3: 0.75%.
Fatty acid profile
The main fatty acid in samples was oleic acid flowed by palmetic and linoleic acids. There were no significant differences among samples in fatty acid composition. Fatty acid profile results confirm that the new salad dressing formulation can be used successfully in the market without concern on fatty acid oxidation (Table 2). The oxidation of fatty acid and their changes were not significant. PV values also confirm these results. PV was not increased at high rate during storage (Table 1).
Table 2. Fatty acid composition (%) for xanthan gum stabilized olive oil-apple cider vinegar salad dressings during storage .
| Parameters | Storage time(days) | Samples | ||
| T 1 | T 2 | T 3 | ||
| Palmitic acid | 1 | 18.2±0.288aA | 18.8±1.27aA | 18.56±0.092aA |
| 90 | 17.9±0.115aA | 17.6±0.288aA | 17.80±0.288aA | |
| Stearic acid | 1 | 2.5±0.144aA | 2.8±0.461aA | 3.06±0.635aA |
| 90 | 3.01±0.011aA | 3.3±0.086aA | 2.6±0.115aA | |
| Oleic acid | 1 | 70.1±0.115aA | 68.9±0.202aA | 69.8±0.493aA |
| 90 | 69.1±0.260aA | 68.2±0.346aA | 71.2±0.404aA | |
| Linoleic acid | 1 | 10.6±0.173aA | 10.86±0.392aA | 10.5±0.404aA |
| 90 | 11.3±0.2.729aA | 11.2±0.288aA | 10.73±0.230aA | |
Mean ± Standard errors. Means within the same row (a, b, c) and the same column (A, B) with different letters are significantly different (p < 0.05). For treatments see Table 1.
Rheological behavior
Flow behavior
The data obtained from the experiments for the shear stress (σ) and shear rate (γ_) at 25 °C were fitted with Power law, Herschel–Bulkley, Bingham and Casson models. The Herschel–Bulkley model with highest coefficients of determination [(R2 = 0.991–0.998)] was chosen as the best model to describe the rheological behavior (Table 3).
Table 3. Rheological parameters from Hershel Balkly and viscoelastic behaviors according frequency sweep test for xanthan gum stabilized olive oil-apple cider vinegar salad dressings .
| Samples | Parameters | ||||||||||||||
| k | n | ɕ 0 (pa) | R | η a 50 | A | Z | |||||||||
|
Storage
time(days) |
1 | 90 | 1 | 90 | 1 | 90 | 1 | 90 | 1 | 90 | 1 | 90 | 1 | 90 | |
| T1 | 0.286±0.003cB | 1.605±0.004bA | 0.866±0.003aA | 0.548±0.004aB | 3.518±0.009cB | 3.653±0.031cA | 0.9962 | 0.9992 | 0.2395±0.0005cB | 0.3468±0.0005cA | 6.2110±0.6072cB | 17.0425±1.2567bA | 0.2429±0.0109aA | 0.2433±0.0118aA | |
| T2 | 3.435±0.003bA | 2.326±0.003aB | 0.433±0.003bB | 0.512±0.011aA | 7.949±0.0527bB | 10.174±0.005bA | 0.9916 | 0.9533 | 0.5326±0.0006bB | 0.5481±0.0006bA | 31.7333±0.9907bB | 36.0888±0.5722aA | 0.1533±0.0054cA | 0.1534±0.0042bA | |
| T3 | 4.845±0.004aA | 1.44±0.020cB | 0.450±0.001cB | 0.608±0.018bA | 18.962±0.051aA | 13.279±0.040aB | 0.9912 | 0.9955 | 0.9425±0.0004aA | 0.5763±0.0006aB | 59.9706±0.8770aA | 35.8374±2368aB | 0.1252±0.0059bA | 0.1333±0.0053bA | |
Mean ± Standard errors. Means within the same row (A, B) and the same column(a, b, c) with different letters are significantly different (P<0.05). For treatments see Table 1.
Trends of shear stress increase with raising the shear rate (Figure 1). In most instances, the flow curve is the first and the main rheological measurement. The value of the yield stress depends on the strength of the absorbing powers holding a droplet together (e.g., the stronger the attracting forces, the higher the yield stress).22 This means that by increasing the amount of xanthan gum in the treatment, the forces between the droplets also increase. In the comparison between the experiments of the first day and 90 days after storage, the charts are closer together and reach equilibrium. This indicates that the structure of T3 firmly declined over time. The structure of T1 is stronger. Over time, the T2 did not show great changes and kept its original structure.
Figure 1.
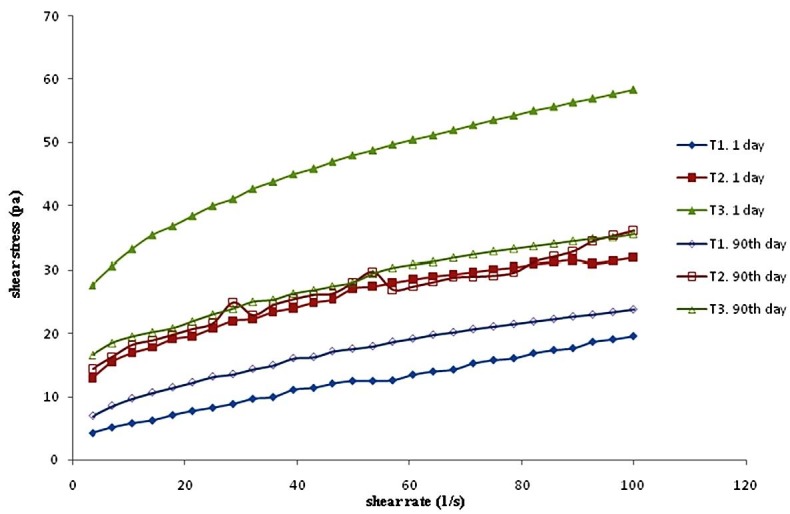
Flow curves of various emulsion samples plotted with shear stress over shear rate for (I) one-day and (II) 90 days. For treatments see Table 1.
The flow behavior indicator (n) is an index employed to recognize the properties of liquid product:
n=1: Newtonian fluid
n < 1 : indicates shear-thinning liquid
n > 1 : shear-thickening or dilatant fluid.
All of the salad dressing samples were fitted with the Herschel–Bulkley models that showed non-Newtonian shear-thinning behavior (non Bingham plastic). None of the samples displayed a linear relation between the shear stress and the shear rate values (Figure 1), exhibiting their pseudoplastic behavior.
The reduction in the apparent viscosity of the samples with time at a constant shear for T1 and T2 was most possible owing to the deformation and/or reformation of oil droplet flocs (Figure 2). The apparent viscosity values of the T1 and T2 (on the first day and after 90 days) at a shear rate of 50 s−1 were in the range of 0.239 to 0.532 Pa s, whereas the T3 (on the first day) was 0.942Pa.s (Table 3). The viscosity value increased as the percent of xanthan in the sauces increased from 0.25 to 0.75 wt.%, thereby lowering the ability of the emulsion to flow. In the emulsion with higher viscosity, the free movement of the oil droplets is delayed, so retarding creaming, flocculation, and coalescence of the oil droplets; our results are consistent with those formerly reported.23
Figure 2.
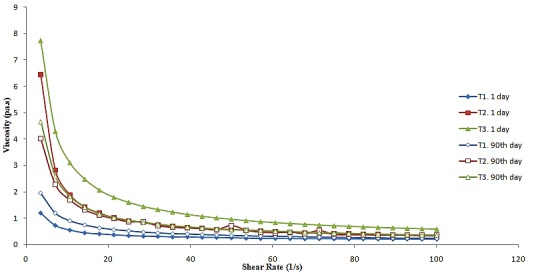
Dependence of viscosity on shear rate for all emulsion samples. For treatments see Table 1.
Furthermore, the K values of O/W emulsion dressings depend on the relation of the oil fraction or the attendance of oil in the emulsion.24 The K index indicates the viscous nature of the emulsion samples (Table 3). Higher K values match up to a stronger emulsion structure. Moreover, the K index for all the salad dressings increases with increasing the percentage of xhanthan for the first day,but on the 90th day K value for T3 sample was lower than T2 sample. This result agrees with the viscosity curve and T3 structure is more stable during shelf life. Samples were not separated into two phases at the end of the storage.
Viscoelastic behaviors
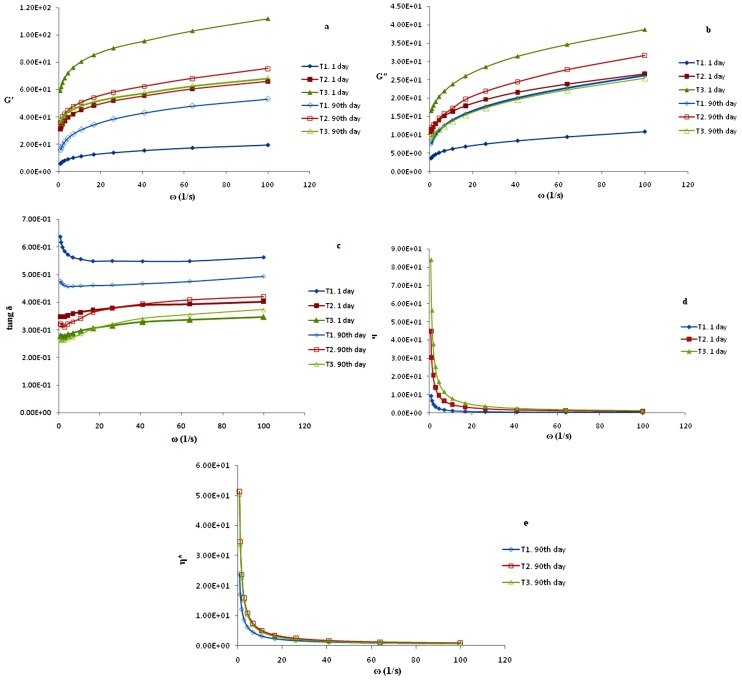
A frequency sweep test is a functional technique for investigating the viscoelastic property of emulsions and is useful in perusing their chemical combination and physical structure.25 The values obtained from this test describe the behavior of the salad dressings and specify if viscouity or elasticity dominates, more than a given frequency range. The frequency dependence of the storage modulus (G′), loss modulus (G″), loss tangent (tan δ), and complex viscosity of all samples on the first day and 90 days after storage is shown in Figure 3a–d. The strength and stability of a material can be specified by several rheological parameters. This information is important for product expansion, mainly for the formulation and processing of emulsions. A graph (Figure 3a) shows the different tendency of G′ in the salad dressings as the frequency increased. G″ also increased in a similar way to G′ in all samples but slightly contrasted with G′ (Figure 3b). The graphs show that all of the sauces prepared with xanthan behave in the same way as gel-like structured materials in which G′ > G″. The results of this study accord with those obtained by the researchers who reported that when G′ > G″, it indicated a gel-like structure with a congested and entwined network, while when G″ > G′, it represented the typical behavior of non-flocculated or weakly flocculated emulsions.18
Figure 3.
(a) torage modulus G′, (b) loss modulus G″, (c) tan δ, (d and e) complex viscosity (η *) versus frequency at first day and after storage at 4 °C for 90-days for all emulsion samples. For treatments see Table 1.
Information concerning the amount of structure present had been demonstrated in a material by the G′ value. T3(with 0.75% of xanthan) on the first day had the highest G′ value, followed by T2 (with 0.5% of gum) after 90days. Effect of the colloidal forces on the interplay between the particles and the droplets is probably the cause of the concentrated O/W systems with higher G′ value.
A measure of the quality of an emulsion's behavior (emulsion strength) is shown by the tangent of the phase angle. In fact, elasticity or viscosity of emulsions is determined with this parameter. Using tan δ is the best manner to demonstrate the strength of an emulsion (for comparison) (Figure 3 c).
Tan δ = G″/ G′ reveals the strength of the colloidal forces. The viscoelastic behavior of the emulsions is described by ratio of the elastic modulus to the loss modulus. Emulsions with (G″/ G′ near 1) have liquid-like or viscous behavior, and materials with (G″/ G′ less than 1) are solid-like or elastic. Particles’ high connection caused by colloidal forces is displayed with tan δ>1, since probably sedimentation happens. The salad dressing with 0.25% xanthan gum on the first day of product had the highest tan δ value, followed by the sample with 0.5% xanthan gum and the emulsion with 0.75% of the same gum, compared with each other. By comparing the results of the first day and the 90th day, it is noted that tan δ declines for all samples, but in the case of T1 and T3, this is significant (p<0.05). The emulsion with 0.75% xanthan gum had the highest values of G′ and tan δ, suggesting that it had the greatest stability during the 90 days of storage in this study, but sauces with 0.5% of gum had the lowest change between the first day and 90th day parameter; as a result, passage of time had the least effect on this sample (Figure 3a,c). These results agreed with those reported by Franco et al, who found out that the tan δ value was related with the emulsion stability, which means that a higher tan δ value was linked with a more stable emulsion product.26 Also, Ma et al suggested that emulsions with a greater fat content demonstrated higher values of G′ because G′ represents the recoverable energy when the material undergoes deformation.26 The results indicate that for the tan δ values of all of the emulsions with xhantan gum, ranging from 0.24 to 0.65, which is <1, all of the emulsion showed solid-like/elastic behaviour rather than viscous behaviour. This conclusion agreed well with that of Steffe et al (1996), who reported that the smaller the tan δ value, the more elastic the system is and the larger the tan δ value, the more fluid the system is.17
The magnitudes of the complex viscosity (η*) decreased with the increase in frequency (Figure 3.d and e).The emulsion with 0.75.% xanthan had the highest η* value (on the first day), followed by those with 0.5.% gum and sample T3 had the lowest value. The system with a higher viscosity could be interrelated to one or more of the following interlinked factors: a thicker adsorbed layer, stronger interactions between the globules, smaller globules, and/or stronger water immobilization by the adsorbed peptides, therefore increasing the thickness of the membranes surrounding fat globules, which consequently resist coalescence; otherwise, rapid destabilization happens, causing oil separation.27
Since the number A represents the power structure, by increasing the percentage of the gum and with the passage of time, the structure of salad dressings are more stable and firm (Table 3).
The Z numbers shown in Table 3 are testament to the determination of the type of structure (non Bingham plastic) for production samples.
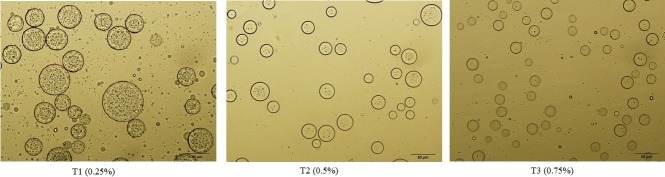
Microstructure
Particle distributions of emulsions containing different concentrations of gum are well visible by microscope (Figure 4).The sample containing 0.25% xanthan had wider particle size distribution and also was larger than the other emulsions.With increasing the percent of xhantan, uniform particle was greater and droplet size was smaller. According to previous studies, increasing in viscosity due to high concentration of gum probably causes the oil droplets to become smaller and unflocculated.20,21,28
Figure 4.
Microstructure and of olive oil– apple cider vinegar functional salad dressings stabilized with xhantan gum at first day of production. For treatments see Table 1.
Droplet size distribution
In describing the particle size, D [4,3] represents volume average particle and span index shows dispersion around the mean. The results show that by increasing gum concentration of 0.25% to 0.75%, all particle size description parameters, including D [4,3] and span index reduced significantly (Table 4).
Table 4. Droplet mean diameter, disparity index (span) and particle size of olive oil– apple cider vinegar salad dressings at first day.
| Samples | Parameters | ||||
| D v10 | D v50 | D v90 | Span index | D 4,3 (µm) | |
| T1 | 2.40±0.05a | 3.5±0.57a | 26±0.57a | 6.74±0.02b | 135.02±0.57a |
| T2 | 2.30±0.11a | 2.8±0.17b | 24 ±1.15b | 7.8±0.16a | 32.51±0.57b |
| T3 | 2.4±0.17a | 3.10±0.11ab | 20±1.73b | 5.67±0.02c | 22.98±0.71c |
Mean ± Standard errors. Means within the same column (a, b, c) with different letters are significantly different (p < 0.05). For treatments see Table 1.
Sensory analysis
According to the scores given by the organoleptic panelists evaluating the samples, there was no significant (P<0.05) difference among the samples (Table 5). But over time, a significant reduction in scores on taste and texture in T1 was observed. In participants, opinion, color change over time was seen only in T1, and there was no significant difference between T2 and T3 treatments. Texture of T1 was not accepted by the panelists and it dropped by extending the shelf life. But the texture of samples T2 and T3 was the same rating and did not change over time. Generally, for the panelists participating in the test samples, T2 and T3 had higher scores and were more acceptable.
Table 5. Mean sensory scores of olive oil– apple cider vinegar salad dressings during storage at 4°C.
| Properties | Storage (days) | Samples | ||
| T 1 | T 2 | T 3 | ||
| Flavour | 1 | 4±0.5aA | 3.5±0.26aA | 4.12±0.12aA |
| 90 | 3.35±0.26aA | 3.35±0.30aA | 3.71±0.32aA | |
| Color | 1 | 4.12±0.47aA | 4.12±0.29aA | 4.5±0.32aA |
| 90 | 3.57±0.27bA | 4.35±0.22aA | 4.28±0.22aA | |
| Texture | 1 | 3.12±0.51aA | 3.75±0.31bA | 4.87±0.12aA |
| 90 | 2.92±0.28bA | 4.57±0.17aA | 4.28±0.24aA | |
| Acceptance | 1 | 11.25±1.42aA | 12.5±0.56aA | 13±0.63aA |
| 90 | 9.85±0.67bA | 12±0.58aA | 12.28±0.57aA | |
Mean ± Standard errors. Means within the same column (a, b, c) and the same row (A, B, C) with different letters are significantly different (p < 0.05). For treatments see Table 1.
Conclusion
In this study, the content of polysaccharide xanthan was investigated to create optimal physicochemical characteristics and oxidative stability and favorable rheological behavior in salad dressings free of cholesterol and egg yolk produced by extra virgin olive oil and apple vinegar. Chemical and rheological results illustrated that among the 3 treatments of different percentages of xanthan gum that were prepared, T2 with 0.5% xanthan gum showed the most desirable oxidative stability and the best rheological behavior, and it gained the highest score of sensory evaluation. Also, comparison of periodic testing states that T2 had the significantly lowest changes during storage. Thus, salad dressing formulation with extra olive oil and apple vinegar with 0.5% xanthan gum can be used in the industrial production and produce functional sauce with optimal physicochemical characteristics, oxidative and rheological and customer-friendly production.
Acknowledgments
Funding for this study was provided by Tabriz University of Medical Sciences and the Department of Food Science and Technology.
Ethical Issues
Not applicable.
Conflict of Interest
The Authors report no declaration of interest.
References
- 1. Sheldrake P. Controlling textures in soups, sauces and dressings. In: McKenna B, editor. Texture in food. Volume 1: Semi-Solid Foods. Cambridge England: woodhead; 2003. P. 389-421.
- 2.Bendini A, Cerretani L, Carrasco-Pancorbo A, Gómez-Caravaca AM, Segura-Carretero A, Fernández-Gutiérrez A. et al. Phenolic molecules in virgin olive oils: a survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade Alessandra. Molecules. 2007;12(8):1679–719. doi: 10.3390/12081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Östman E, Granfeldt Y, Persson L, Björck I. Vinegar supplementation lowers glucose and insulin responses and increases satiety after a bread meal in healthy subjects. Eur J Clin Nutr. 2005;59(9):983–8. doi: 10.1038/sj.ejcn.1602197. [DOI] [PubMed] [Google Scholar]
- 4.Johnston CS, Kim CM, Buller AJ. Vinegar improves insulin sensitivity to a high-carbohydrate meal in subjects with insulin resistance or type 2 diabetes. Diabetes Care. 2004;27(1):281–2. doi: 10.2337/diacare.27.1.281. [DOI] [PubMed] [Google Scholar]
- 5.Dickinson E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. 2003;17(1):25–39. doi: 10.1016/S0268-005X(01)00120-5. [DOI] [Google Scholar]
- 6.Lai L, Lin P. Application of decolourised hsian-tsao leaf gum to low-fat salad dressing model emulsions: a rheological study. J Sci Food Agric. 2004;84(11):1307–14. doi: 10.1002/jsfa.1757. [DOI] [Google Scholar]
- 7.Garcıa-Ochoa F, Santos VE, Casas JA, Gomez E. Xanthan gum: production, recovery, and properties. Biotechnol Adv. 2000;18(7):549–79. doi: 10.1016/S0734-9750(00)00050-1. [DOI] [PubMed] [Google Scholar]
- 8.Katzbauer B. Properties and applications of xanthan gum. Polym Degrad Stab. 1998;59(1-3):81–4. doi: 10.1016/S0141-3910(97)00180-8. [DOI] [Google Scholar]
- 9.Sun C, Gunasekaran S, Richards MP. Effect of xanthan gum on physicochemical properties of whey protein isolate stabilized oil-in-water emulsions. Food Hydrocoll. 2007;21(4):555–64. doi: 10.1016/j.foodhyd.2006.06.003. [DOI] [Google Scholar]
- 10.McClements DJ, Decker EA. Lipid oxidation in oil-in-water emulsions: Impact of molecular environment on chemical reactions in heterogeneous food systems. J Food Sci. 2000;65(8):1270–82. doi: 10.1111/j.1365-2621.2000.tb10596.x. [DOI] [Google Scholar]
- 11.Shimada K, Okada H, Matsuo K, Yoshioka S. Involvement of chelating action and viscosity in the antioxidative effect of xanthan in an oil/water emulsion. Biosci Biotechnol Biochem. 1996;60(1):125–7. [Google Scholar]
- 12.Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40(6):945–8. doi: 10.1021/jf00018a005. [DOI] [Google Scholar]
- 13.Paraskevopoulou D, Boskou D, Paraskevopoulou A. Oxidative stability of olive oil-lemon juice salad dressings stabilized with polysaccharides. Food Chem. 2007;101(3):1197–204. doi: 10.1016/j.foodchem.2006.03.022. [DOI] [Google Scholar]
- 14.Jacobsen C, Hartvigsen K, Lund P, Meyer AS, Adler-Nissen J, Holstborg J. et al. Oxidation in fish-oil-enriched mayonnaise 1. Assessment of propyl gallate as an antioxidant by discriminant partial least squares regression analysis. Eur Food Res Technol. 1999;210(1):13–30. doi: 10.1007/s002170050526. [DOI] [Google Scholar]
- 15.Azadmard-Damirchi S, Dutta PC. Novel solid-phase extraction method to separate 4-desmethyl-, 4-monomethyl-, and 4,4'-dimethylsterols in vegetable oils. J Chromatogr A. 2006;1108(2):183–7. doi: 10.1016/j.chroma.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Ng SP, Lai OM, Abas F, Lim HK, Tan CP. Stability of a concentrated oil-in-water emulsion model prepared using palm olein-based diacylglycerol/virgin coconut oil blends: Effects of the rheological properties, droplet size distribution and microstructure. Food Res Int. 2014;64:919–30. doi: 10.1016/j.foodres.2014.08.045. [DOI] [PubMed] [Google Scholar]
- 17.Steffe JF. Rheological methods in food process engineering. 2nd ed. USA: Freeman Press; 1996. [Google Scholar]
- 18.Ma Z, Boye JI. Advances in the design and production of reduced-fat and reduced-cholesterol salad dressing and mayonnaise: a review. Food Bioprocess Technol. 2013;6(3):648–70. doi: 10.1007/s11947-012-1000-9. [DOI] [Google Scholar]
- 19.Bortnowska G, Balejko J, Schube V, Tokarczyk G, Krzemińska N, Mojka K. Stability and physicochemical properties of model salad dressings prepared with pregelatinized potato starch. Carbohydr Polym. 2014;111:624–32. doi: 10.1016/j.carbpol.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Karimi N, Mohammadifar M, Nayebzade K. Effect of two types of Iranian gum tragacanth on stability and rheological properties of oil-in-water emulsion. Iran J Nutr Sci Food Technol. 2013;8(3):87–98. [Google Scholar]
- 21.Mei L, Decker EA, McClements DJ. Evidence of iron association with emulsion droplets and its impact on lipid oxidation. J Agric Food Chem. 1998;46(12):5072–7. doi: 10.1021/jf9806661. [DOI] [Google Scholar]
- 22.Pal R. Viscosity and storage/loss moduli for mixtures of fine and coarse emulsions. Chem Eng J. 1997;67(1):37–44. doi: 10.1016/S1385-8947(97)00011-9. [DOI] [Google Scholar]
- 23.Tesch S, Schubert H. Influence of increasing viscosity of the aqueous phase on the short-term stability of protein stabilized emulsions. J Food Eng. 2002;52(3):305–12. doi: 10.1016/S0260-8774(01)00120-0. [DOI] [Google Scholar]
- 24.Chatsisvili NT, Amvrosiadis I, Kiosseoglou V. Physicochemical properties of a dressing-type o/w emulsion as influenced by orange pulp fiber incorporation. LWT-Food Sci Technol. 2012;46(1):335–40. doi: 10.1016/j.lwt.2011.08.019. [DOI] [Google Scholar]
- 25.Cruz M, Freitas F, Torres CA, Reis MA, Alves VD. Influence of temperature on the rheological behavior of a new fucose-containing bacterial exopolysaccharide. Int J Biol Macromol. 2011;48(4):695–9. doi: 10.1016/j.ijbiomac.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Franco JM, Guerrero A, Gallegos C. Rheology and processing of salad dressing emulsions. Rheol Acta. 1995;34(6):513–24. doi: 10.1007/BF00712312. [DOI] [Google Scholar]
- 27.Ma L, Barbosa-Cánovas G. Rheological characterization of mayonnaise. Part II: Flow and viscoelastic properties at different oil and xanthan gum concentrations. J Food Eng. 1995;25(3):409–25. doi: 10.1016/0260-8774(94)00010-7. [DOI] [Google Scholar]
- 28.Khouryieh H, Puli G, Williams K, Aramouni F. Effects of xanthan-locust bean gum mixtures on the physicochemical properties and oxidative stability of whey protein stabilised oil-in-water emulsions. Food Chem. 2015;167:340–8. doi: 10.1016/j.foodchem.2014.07.009. [DOI] [PubMed] [Google Scholar]