Abstract
REC8 meiotic recombination protein (REC8) was found to be preferentially methylated in gastric cancer (GC) using promoter methylation array. We aimed to elucidate the epigenetic alteration and biological function of REC8 in GC. REC8 was downregulated in 100% (3/3) of Epstein–Barr virus (EBV)-positive and 80% (8/10) of EBV-negative GC cell lines by promoter methylation, but the expression could be restored through demethylation treatment. Protein expression of REC8 was significantly lower in human primary gastric tumors than in adjacent non-tumor tissues. A negative correlation between methylation and mRNA expression of REC8 was observed in 223 gastric samples of The Cancer Genome Atlas study (r=−0.7018, P<0.001). The methylation level (%) of the REC8 promoter was significantly higher in EBV-positive gastric tumors than in EBV-negative gastric tumors, as shown by bisulfite genomic sequencing (77.6 (69.3–80.5) vs 51.4 (39.5–62.3), median (interquartile range); P<0.001); methylation levels in both subtypes of tumors were significantly higher than in normal stomach tissues (14.8 (4.2–24.0)) (both P<0.001). Multivariate analysis revealed that REC8 methylation was an independent factor for poor survival in GC patients (hazard ratio=1.68, P<0.05). REC8 expression significantly suppressed cell viability, clonogenicity and cell cycle progression; it induced apoptosis and inhibited migration of AGS-EBV (EBV-positive) and BGC823 (EBV-negative) GC cells, and it suppressed tumorigenicity in nude mice. In contrast, knockdown of REC8 in gastric epithelial immortalized GES-1 cells significantly increased cell viability, clonogenicity and migration ability. The tumor-suppressive effect of REC8 is mediated at least in part by the downregulation of genes involved in cell growth (G6PD, SLC2A1, NOL3, MCM2, SNAI1 and SNAI2), and the upregulation of apoptosis/migration inhibitors (GADD45G and LDHA) and tumor suppressors (PinX1, IGFBP3 and ETS2). In conclusion, REC8 is a novel tumor suppressor that is commonly downregulated by promoter methylation in GC, especially in the EBV-associated subtype. Promoter methylation of REC8 is an independent risk factor for the shortened survival of GC patients.
Introduction
Gastric cancer (GC) is one of the most common malignant tumors and remains the third leading cause of cancer-related death worldwide.1 Evidence from us and others has shown that epigenetic alterations, particularly the inactivation of tumor-suppressor genes by promoter hypermethylation, have an important role in the occurrence and development of GC.2, 3, 4, 5 Epstein–Barr virus (EBV)-associated GC is a characteristic subtype of GC, showing distinct clinicopathological features compared with EBV-negative ones.6 Genome-wide EBV-associated DNA hypermethylation has been revealed in GC by comparing EBV-positive and -negative GCs.7, 8, 9 Some genes, such as PTEN, CDH1, APC, SSTR1, TRABD and IHH, have been shown to be more frequently methylated in EBV-positive GC than in EBV-negative GC and to have tumor-suppressive functions.9, 10, 11, 12
In a previous study, we compared the genome-wide methylation profiles between the GC cell line with stable EBV infection (AGS-EBV) and its parental uninfected AGS cells using the methylated DNA immunoprecipitation microarray (MeDIP-chip).7 The promoter of the gene, REC8 meiotic recombination protein (REC8), was found to be methylated 23 times more in AGS-EBV cells than in AGS cells. Further expressional screening revealed that REC8 was downregulated or silenced in most GC cell lines, including both EBV-positive and -negative GC cells. REC8 belongs to the cohesin protein complex, which is essential for correct chromosome disjunction and homologous recombination in the mitotic and meiotic cycle.13 In thyroid cancer, REC8 has been shown to be a tumor-suppressor gene that is epigenetically regulated and robustly targeted by the phosphoinositide-3 kinase pathway.14 Aberrant methylation of REC8 has also been detected in the brain tissues of patients with schizophrenia.15 REC8 methylation has been found in small and malignant gastrointestinal stromal tumors, and patients with methylation of REC8, PAX3, or CDKN2A had a significantly poorer prognosis.16 However, the role of REC8 in GC remains elusive. Therefore, this study is aimed to elucidate the expression and epigenetic regulation of REC8 in GC, with particular attention to the EBV subtype of GC. The biological function and molecular mechanism of REC8, as well as the clinical implication of its promoter methylation, were further investigated.
Results
REC8 was downregulated in GC cells by promoter methylation
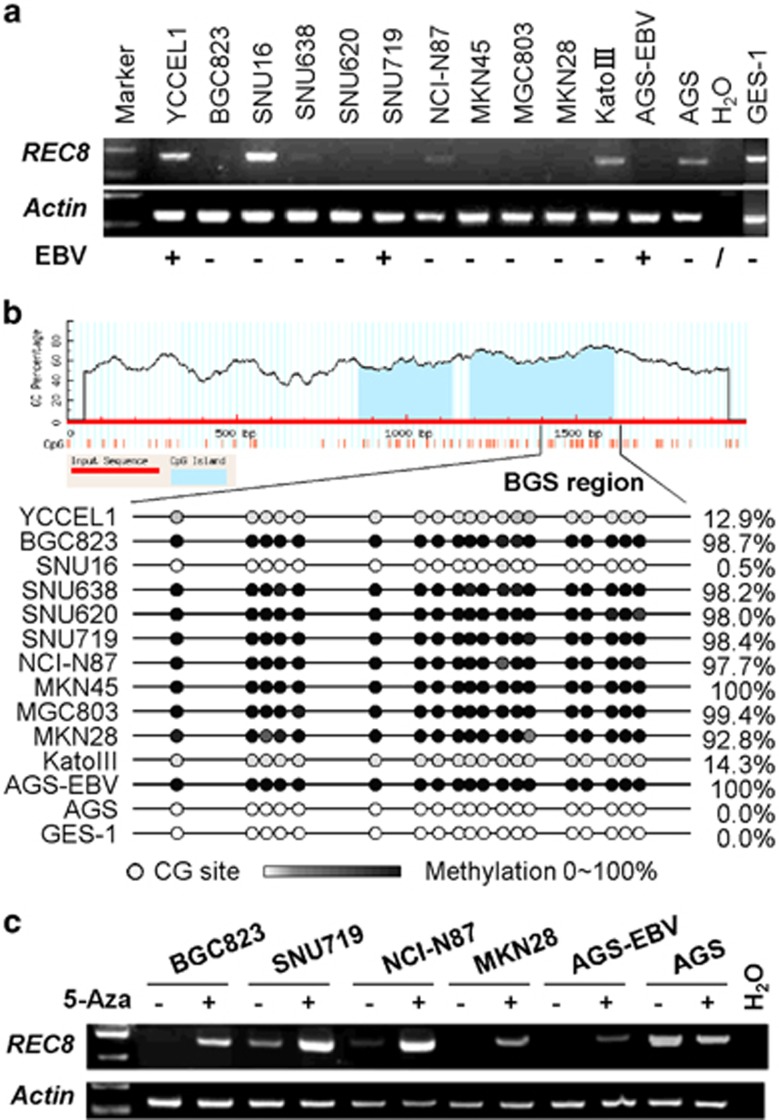
REC8 was downregulated or silenced in 69.2% (9/13) of GC cell lines, including 3 EBV-positive and 10 EBV-negative, as indicated by reverse transcription PCR (RT–PCR) (Figure 1a). We then characterized the methylation status of the REC8 promoter region by bisulfite genomic sequencing (BGS). Results showed that the REC8 downregulation or silencing was correlated with its promoter methylation. All nine cell lines with silenced REC8 (BGC823, SNU638, SNU620, SNU719, NCI-N87, MKN45, MGC803, MKN28 and AGS-EBV) showed full promoter methylation, while other cells with active REC8 expression (YCCEL1, SNU16, KatoIII, AGS and GES-1) showed no or only partial methylation (Figure 1b). We further treated six cell lines with the DNA demethylation agent 5-Aza-2'-deoxycytidine (5-Aza). REC8 mRNA expression was restored in the five cell lines with REC8 downregulated/methylated (BGC823, SNU719, NCI-N87, MKN28 and AGS-EBV) but had no effect in AGS with REC8 expression/unmethylated (Figure 1c), further supporting the hypothesis that the transcriptional silencing of REC8 is mediated by promoter methylation. These results collectively demonstrated that the expression of REC8 is mainly regulated by promoter methylation in GC cells.
Figure 1.
Transcriptional silencing of REC8 in GC is associated with DNA methylation. (a) REC8 expression was determined by RT–PCR. REC8 (normalized to β-actin) was silenced in 9 of the 13 detected GC cell lines. (b) BGS analysis confirmed the methylation status of REC8 in GC cell lines. (c) The mRNA expression of REC8 was restored after treatment with demethylation agent, 5-Aza.
Promoter of REC8 was hypermethylated in primary gastric tumors, especially the EBV-positive subtype
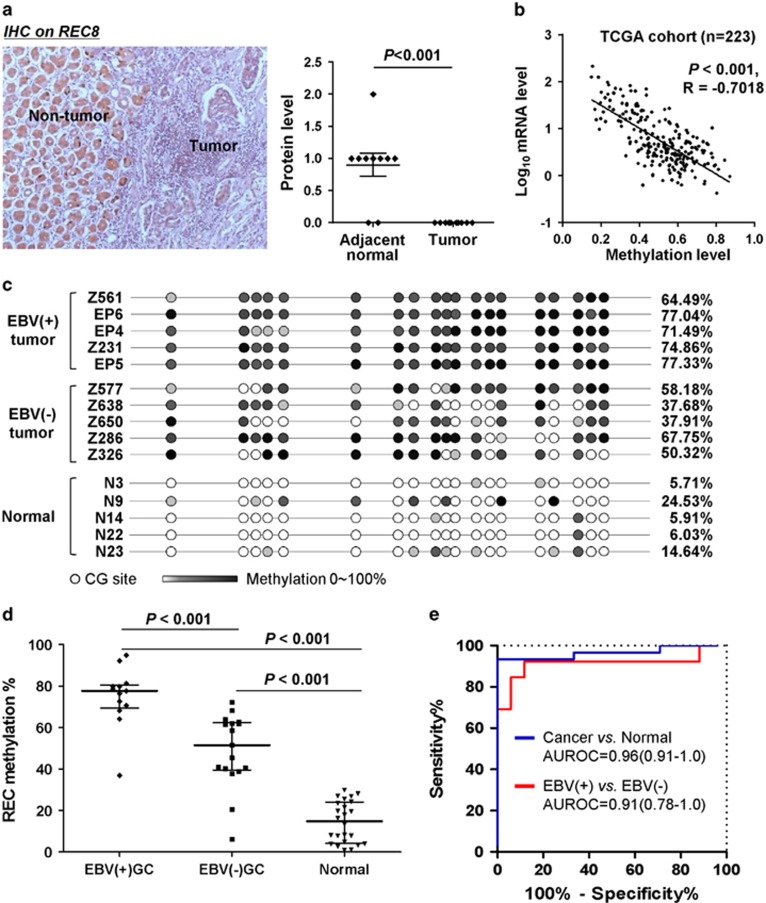
As REC8 was downregulated in both EBV-positive and -negative GC cell lines, we examined the expression and promoter methylation of REC8 in both EBV-positive and -negative primary GCs. Immunohistochemistry analysis showed that REC8 protein expression was significantly reduced in gastric tumors as compared with adjacent non-tumor tissues of Chinese patients (Figure 2a). We retrieved methylation and mRNA expression data on REC8 from 223 gastric samples available in The Cancer Genome Atlas (TCGA) database. Linear regression analysis demonstrated a significant negative correlation between REC8 promoter methylation and mRNA expression (R=−0.7018, P<0.001; Figure 2b), suggesting a pivotal regulatory role of promoter methylation on REC8 expression in GC. We further compared the methylation levels of REC8 in EBV-positive, EBV-negative primary gastric tumors and normal stomach mucosa of Chinese subjects by BGS (Figure 2c). REC8 promoter was methylated at significantly higher levels in both EBV-positive gastric tumors (74.8±3.9%, n=13) and EBV-negative gastric tumors (48.9±4.3%, n=18) as compared with normal stomach mucosa (14.4±2.0%, n=24) (both P<0.001); the methylation level was significantly higher in EBV-positive tumors than in EBV-negative tumors (P<0.001) (Figure 2d). Receiver Operating Characteristic (ROC) curve analysis indicated that a cutoff value of 33.4% REC8 methylation could discriminate gastric tumors from normal mucosa with a sensitivity and specificity of 93.3% and 100%, respectively (area under the ROC curve (AUC)=0.96 (0.91–1.0)), and a cutoff value of 68.1% could discriminate EBV-positive tumors from EBV-negative tumors with a sensitivity and specificity of 84.6% and 94.1%, respectively (AUC=0.91 (0.78–1.0)) (Figure 2e). In all 31 cancer patients (13 EBV-positive and 18 EBV-negative), linear regression analysis showed that REC8 methylation was significantly associated with positive EBV-encoded RNA (EBER) staining/EBV positivity (linear coefficient: 23.73, 95% confidence interval: 10.72–36.74; P=0.001) but not with other clinicopathological features, such as age, gender, Helicobacter pylori infection, histological type, differentiation and tumor, node, metastasis (TNM) staging (Table 1). REC8 is frequently methylated in GCs, with EBV infection being one of the important factors for the high methylation level of REC8 promoter.
Figure 2.
Reduced REC8 expression by promoter methylation in primary gastric tumors. (a) REC8 protein expression was observed in non-tumor normal tissues but not detected in gastric tumor tissues of Chinese patients by immunohistochemistry. (b) A negative correlation between promoter methylation and mRNA levels of REC8 in the TCGA cohort of 223 gastric samples. R, correlation coefficient. (c and d) Promoter methylation level of REC8 was determined in primary EBV-positive and -negative GCs and normal stomach tissues of Chinese patients by bisulfite genomic sequencing. (e) ROC curve analysis showed that methylation level of REC8 could discriminate between GC and normal mucosa, as well as EBV-positive and -negative gastric tumors.
Table 1. Linear regression analysis of potential predictor for REC8 methylation in gastric cancer patients (with EBV status).
| Variable | β | P-value |
|---|---|---|
| Age | 0.404 | 0.215 |
| Gender | 0.706 | |
| Male | 3.316 | |
| Female | 1 | |
| H. pylori | 0.493 | |
| Negative | −5.394 | |
| Positive | 1 | |
| Lauren | 0.057 | |
| Non-intestinal | 19.292 | |
| Intestinal | 1 | |
| Differentiation | 0.342 | |
| Moderate or high | −7.592 | |
| Low | 1 | |
| TNM stage | ||
| I | −5.354 | 0.783 |
| IIa | NA | NA |
| III | −8.660 | 0.709 |
| IV | 1 | |
| EBER | 0.001 | |
| Positive | 23.727 | |
| Negative | 1.00 |
Abbreviations: EBER, EBV-encoded RNA; EBV, Epstein–Barr virus; NA, not available; TNM, tumor, node, metastasis. Linear regression analysis was performed on 31 gastric cancer patients, including 13 EBV-positive and 18 EBV-negative.
No case of TNM stage II was included. P-value <0.05 is denoted in bold type.
Clinicopathological features of REC8 promoter methylation in GC patients
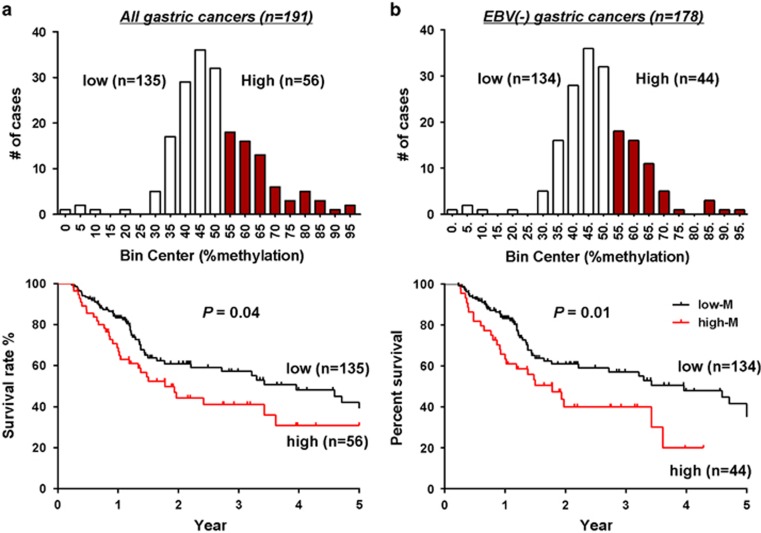
In order to delineate the clinical implications of REC8 promoter methylation in GC patients, we quantitated the promoter methylation levels of REC8 by BGS in 191 Chinese GC patients with follow-up data (median survival time: 1.24 years). Analysis of survival data was limited within a 5-year period to avoid the probability of death not owing to GC. The cutoff value of 33.4% was calculated using ROC curve analysis as the level that maximizes the sum of sensitivity and specificity in discriminating cancer from normal mucosa. Using this level, we found REC8 to be methylated in 94.2% (180 of 191) cases of primary GCs. Using Cutoff Finder for survival significance analysis,17 we classified the 191 GCs as low- and high-methylated using the cutoff value of 55% the distribution histogram of which is shown in Figure 3a. As shown in the Kaplan–Meier survival curves, Chinese GC patients with high methylation of REC8 promoter, regardless of EBV infection status, had a significantly shortened survival than those with low methylation (P=0.04, log-rank test; Figure 3a). There was no correlation between low/high methylation of REC8 and clinicopathological features, such as age, gender, H. pylori infection status, Lauren type, differentiation and TNM stage (Table 2). As an increase in survival was reported in patients with EBV-positive GC compared with those of EBV-negative,6 we performed survival analysis on EBV-negative GCs (n=178). As expected, high methylation of REC8 promoter was significantly associated with shortened survival of patients with EBV-negative GC (P=0.01; Figure 3b), which was more obvious than in all patients (Figure 3a).
Figure 3.
Promoter methylation of REC8 in Chinese GC patients. (a) The distribution of the methylation levels in all 191 GC samples of Chinese patients. High methylation level of REC8 (>55%) significantly correlates with shortened survival in Chinese GC patients. (b) The distribution of the methylation levels in 178 EBV-negative GCs. High methylation level of REC8 (>55%) significantly correlates with shortened survival in EBV-negative GC patients.
Table 2. Distribution of patient characteristics by methylation status (all gastric cancer).
| Variable | High methylation (n=56) | % | Low methylation (n=135) | % | P-value |
|---|---|---|---|---|---|
| Mean age, years±s.d. | 55.89±13.218 | 56.82±12.799 | 0.655 | ||
| Gender | 0.847 | ||||
| M | 36 | 28.3 | 91 | 71.7 | |
| F | 19 | 29.7 | 45 | 70.3 | |
| H. pylori | 0.400 | ||||
| Positive | 34 | 32.1 | 72 | 67.9 | |
| Negative | 21 | 25.0 | 63 | 75.0 | |
| TNM | 0.149 | ||||
| I | 11 | 35.5 | 20 | 64.5 | |
| II | 5 | 20.8 | 19 | 79.2 | |
| III | 19 | 22.1 | 67 | 77.9 | |
| IV | 11 | 36.7 | 19 | 63.3 | |
| Lauren | 0.094 | ||||
| Intestinal | 43 | 26.2 | 121 | 73.8 | |
| Non-intestinal | 12 | 46.2 | 14 | 53.8 | |
| Differentiation | 0.714 | ||||
| Low | 33 | 31.1 | 73 | 68.9 | |
| Moderate or high | 17 | 25.4 | 50 | 74.6 |
Abbreviations: F, female; M, male; TNM, tumor, node, metastasis.
High methylation of REC8 promoter is an independent predictor of poor outcome in patients with GC
Using univariate and multivariate Cox regression analyses, only REC8 methylation status and tumor stage, but not other clinicopathological features (age, gender, H. pylori infection status, Lauren type, differentiation), were found to be significantly associated with the poor survival of all the 191 Chinese GC patients (Table 3). Multivariate Cox regression analysis showed that high methylation of the REC8 promoter, as an independent predictor of poor survival in GC patients, showed a hazard ratio (HR) of 1.68 (P<0.05). When only EBV-negative GCs (n=178) were considered, similarly only REC8 methylation status and tumor stage were found to be significantly associated with shortened patient survival, with an increased HR (1.86) as revealed by multivariate Cox regression analysis for REC8 methylation (Table 3).
Table 3. Univariate and multivariate Cox regression analyses of potential poor prognostic factors for gastric cancer patients (all and EBV-negative gastric cancers).
| Variable |
All 191 cases |
178 EBV-negative cases |
||||||
|---|---|---|---|---|---|---|---|---|
|
Univariate |
Multivariate |
Univariate |
Multivariate |
|||||
| HR | P-value | HR | P-value | HR | P-value | HR | P-value | |
| Age | 0.994 | 0.518 | 0.993 | 0.445 | ||||
| Gender | 0.954 | 0.729 | ||||||
| Male | 1.013 | 1.086 | ||||||
| Female | 1 | 1 | ||||||
| H. pylori | 0.501 | 0.733 | ||||||
| Positive | 1.573 | 1.085 | ||||||
| Negative | 1 | 1 | ||||||
| Lauren | 0.939 | 0.102 | ||||||
| Intestinal | 0.004 | 0.616 | ||||||
| Non-intestinal | 1 | 1 | ||||||
| Differentiation | 0.137 | 0.994 | ||||||
| Low | 1.366 | 1.002 | ||||||
| Moderate/high | 1 | 1 | ||||||
| TNM stage | ||||||||
| I | 0.217 | <0.001 | 0.228 | <0.001 | 0.199 | <0.001 | 0.206 | <0.001 |
| II | 0.084 | <0.001 | 0.087 | <0.001 | 0.090 | <0.001 | 0.076 | <0.001 |
| III | 0.280 | <0.001 | 0.301 | <0.001 | 0.291 | <0.001 | 0.290 | <0.001 |
| IV | 1 | 1 | 1 | 1 | ||||
| REC8 methylation | 0.030 | 0.042 | 0.014 | 0.029 | ||||
| Yes | 1.642 | 1.678 | 1.829 | 1.860 | ||||
| No | 1 | 1 | 1 | 1 | ||||
Abbreviations: EBV, Epstein–Barr virus; HR, hazard ratio; TNM, tumor, node, metastasis. P-values <0.05 are denoted in bold type.
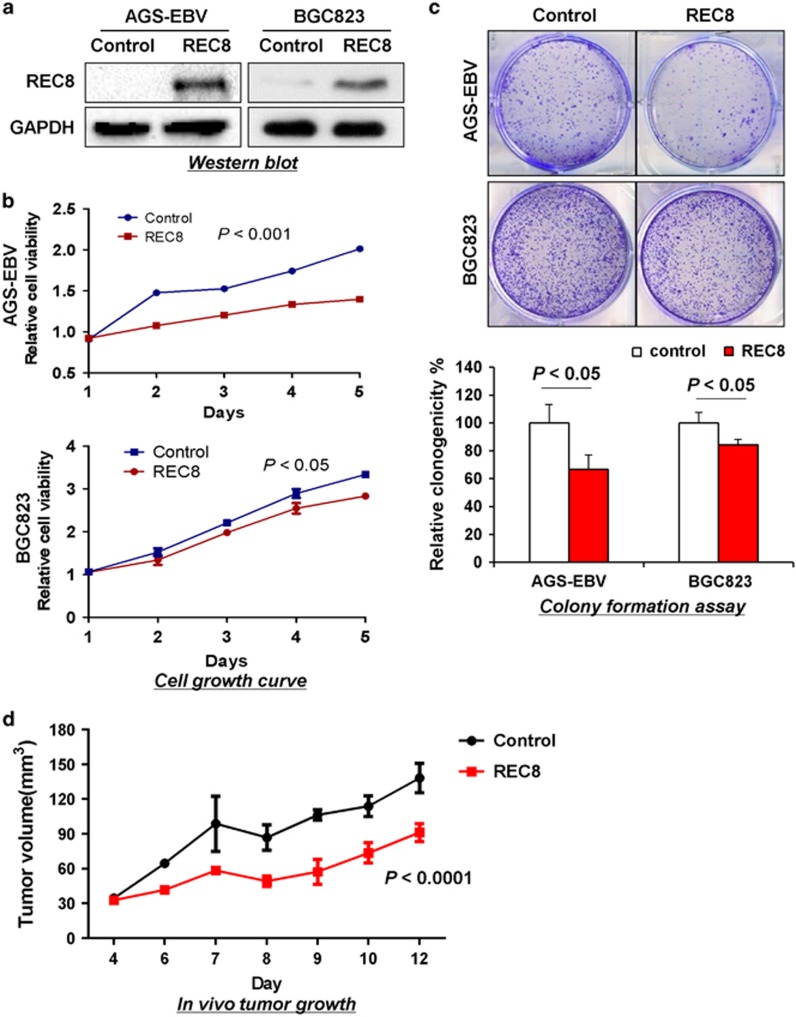
REC8 exerted an inhibitory effect on GC growth
To investigate the role of REC8 in GC, EBV-positive AGS-EBV and EBV-negative BGC823 cells were transfected with REC8 expression vector or empty control vector. Overexpression of REC8 was confirmed by western blotting (Figure 4a). REC8 expression significantly suppressed cell viability of both cell lines as compared with the empty vector transfection (P<0.05; Figure 4b). The growth-suppressive effect of REC8 was further confirmed by the significantly reduced colony-formation ability of REC8-overexpressed cells as compared with controls (P<0.05; Figure 4c). Moreover, REC8 expression was found to significantly suppress tumorigenicity in vivo (P<0.05; Figure 4d). These results demonstrated that REC8 might function as a tumor suppressor in GC.
Figure 4.
REC8 overexpression inhibits the growth of GC cells. (a) Ectopic expression of REC8 in AGS-EBV and BGC823 cell lines was confirmed by western blotting. (b) Overexpression of REC8 significantly reduced cell viability in GC cells. (c) Overexpression of REC8 significantly suppressed colony formation. (d) REC8 significantly attenuated tumorigenicity of BGC823 cells in nude mice.
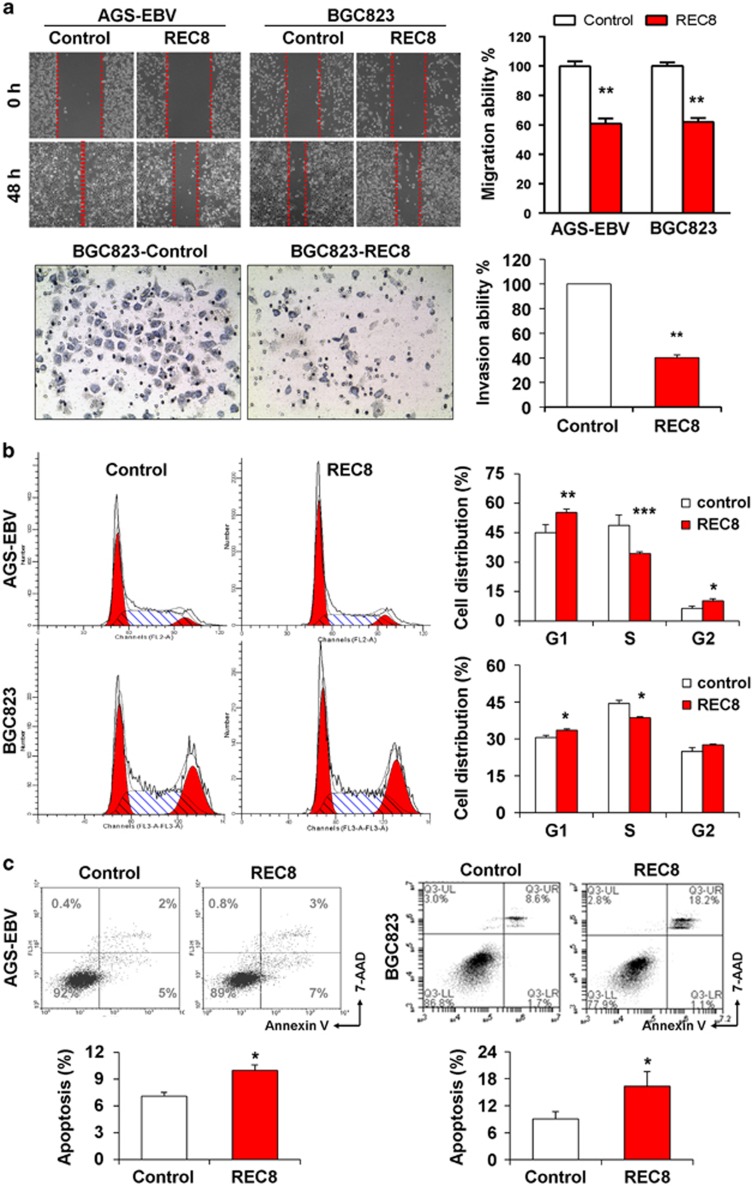
REC8 suppressed migration and invasion ability, inhibited cell cycle progression and induced apoptosis in GC cells
To further understand the tumor-suppressive role of REC8, we next investigated the effect of REC8 overexpression on GC cell migration using the monolayer scratch-healing assay and Matrigel invasion assay. Overexpression of REC8 markedly slowed the migration of AGS-EBV and BGC823 cells. Significant reduction in wound closure was observed at 48 h in REC8-overexpressed cells as compared with control cells for both cell lines (P<0.001; Figure 5a). In addition, REC8 also significantly impaired the invasiveness of BGC823 cells. These results demonstrated an inhibitory effect of REC8 on GC cell migration and invasion.
Figure 5.
REC8 suppressed the migration ability and cell cycle progression and induced apoptosis in GC cells. (a) Representative result of scratch healing and invasion assays. (b) Expression of REC8 increased G1-phase cell population but decreased S-phase cell population, as shown by flow cytometric analysis. (c) REC8 induced apoptosis in GC cells, as determined by flow cytometric analysis following Annexin V and 7-AAD staining. The experiments were performed three times independently. Data are mean±s.d. *P<0.05; **P<0.001; ***P<0.0001.
We further analyzed the effect of REC8 on cell cycle distribution and apoptosis by flow cytometry after propidium iodide staining or dual staining with viability dye 7-aminoactinomycin D (7-AAD) and Annexin V-fluorescein isothiocyanate. Ectopic expression of REC8 led to a significant increase of cells in G1 phase (P<0.05) and a significant reduction of those in S phase (P<0.05) in both AGS-EBV and BGC823 cells (Figure 5b). Expression of REC8 also significantly increased the apoptotic cell proportion in both cell lines (P<0.05; Figure 5c). These results demonstrated that REC8 suppressed GC cell growth via inhibiting cell cycle progression and inducing cell apoptosis.
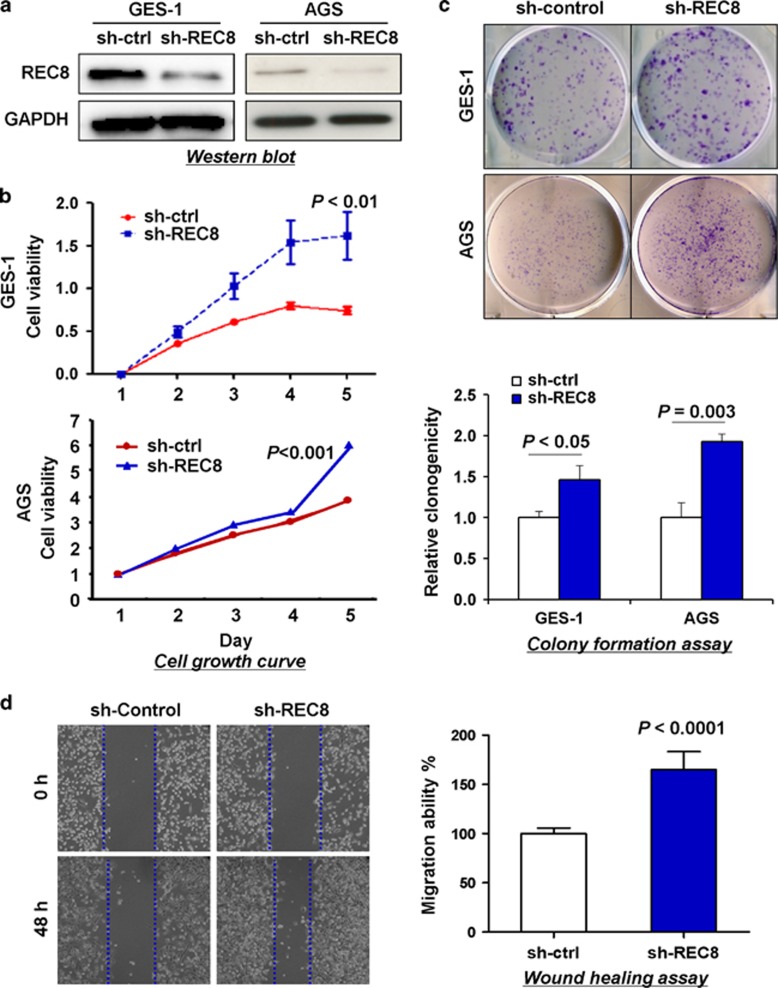
Knockdown of REC8 promoted cell growth and migration
To further confirm the inhibitory effect of REC8 on GC cell growth, we knocked down REC8 expression in the immortalized human gastric epithelial mucosa cell line, GES-1, and the GC cell line, AGS, which showed high endogenous expression of REC8. Successful knockdown of REC8 was confirmed by western blotting after stable transfection of the short hairpin RNA (shRNA)-REC8 vectors (Figure 6a). Knockdown of REC8 significantly increased cell viability in both GES-1 and AGS cells as compared with their controls transfected with scrambled-shRNA vectors (P<0.01; Figure 6b). The growth-enhancing effect of REC8 knockdown was further supported by colony-formation assay. The colonies formed by shRNA-REC8-transfected cells were significantly more in number and larger in size as compared with those formed by control cells (P<0.05; Figure 6c). Stable knockdown of REC8 also markedly accelerated cell migration at the edges of scratch wounds (Figure 6d). Quantitative analysis at 48 h indicated a significant increase in the migration ability of GES-1 cells transfected with shRNA-REC8 compared with the control cells (P<0.05). These promoting effects on cell growth and migration by knockdown of REC8 further confirmed the tumor-suppressive role of REC8 in GC.
Figure 6.
REC8 knockdown increased GC cell growth and migration. (a) Knockdown of REC8 was confirmed at the protein level in AGS and GES-1 cells by western blotting. (b) REC8 knockdown significantly increased cell viability. (c) REC8 knockdown significantly promoted colony formation. (d) Knockdown of REC8 enhanced GES-1 cell migration ability, as shown by wound-healing assay. All experiments were performed three times independently. Data are mean±s.d.
Identification of genes modulated by REC8
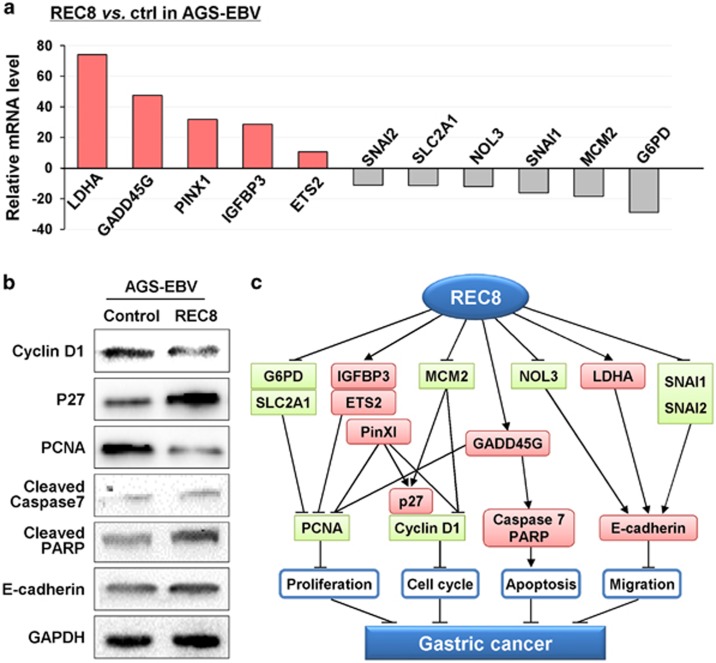
To gain insight into the molecular mechanism underlying the tumor-suppressive effect of REC8, the expression of 84 pivotal genes involved in cancer pathways were analyzed by Human Cancer Pathway complementary DNA (cDNA) array in AGS-EBV cells with/without REC8 expression. REC8 was found to modulate the expression of important genes involved in multiple cellular processes, including proliferation, cell cycle, apoptosis, migration and differentiation (Figure 7a). REC8 inhibited the cell proliferation regulators (G6PD (glucose-6-phosphate dehydrogenase) and SLC2A1) and apoptosis inhibitor (NOL3), while inducing the expression of the apoptosis regulator (GADD45G) and tumor suppressors (PinX1 (PIN2/TRF1-interacting telomerase inhibitor1), IGFBP3 (insulin-like growth factor-binding protein 3) and ETS2), which may ultimately inhibit cell proliferation and increase cell apoptosis. REC8 also inhibited the cell cycle G1–S transition-promoting regulator, MCM2, which may subsequently mediate cell cycle arrest at G1–S transition. Moreover, REC8 reduced the expression of two epithelial–mesenchymal transition promoters (SNAI1 and SNAI2) and induced the migration inhibitor lactate dehydrogenase A (LDHA), resulting in inhibited cell migration ability.
Figure 7.
Molecular mechanism of the tumor-suppressive role of REC8 in GC. (a) Downstream effectors of REC8 were identified by Human cancer pathway PCR array. (b) Expression of apoptotic, proliferative, migratory and cell cycle-related genes was evaluated by western blotting. GAPDH was used as a loading control. (c) Schematic diagram of the molecular events for REC8's function as a tumor suppressor through regulating cell cycle, proliferation, apoptosis and migration effectors.
We also examined some important effectors of proliferation, cell cycle and migration by western blotting. Results showed that REC8 suppressed the G1–S transition promoter (cyclin D1) and the proliferation marker (proliferating cell nuclear antigen (PCNA)), and it induced the G1 gatekeeper (p27), supporting the effect of REC8 on blocking cell cycle progression at the G1–S checkpoint. Consistent with increased apoptosis and reduced cell migration ability by REC8, upregulation of pro-apoptotic regulators (cleaved-caspase 7 and cleaved-PARP (poly (ADP-ribose) polymerase)) and the cell-to-cell adhesion gene (E-cadherin) were also detected (Figure 7b). The correlations between REC8 and its downstream targets, as well as their association with the inhibition of GC growth, are shown in Figure 7c. These results unveiled the molecular mechanism by which REC8 had a tumor-suppressive role in GC.
Discussion
In this study, we confirmed that REC8 was downregulated in GC, especially in the EBV-associated subtype, via promoter methylation. Clinical implications of REC8 promoter methylation and functional importance of REC8 as a novel tumor suppressor in GC were also elucidated.
We have previously investigated EBV-associated host gene methylation in GC; REC8 was identified to be one of the hypermethylated genes in EBV-positive GC cell line (AGS-EBV) compared with EBV-negative AGS cell line. We further validated the methylation level of REC8 promoter to be significantly higher in EBV-associated gastric tumors as compared with EBV-negative gastric tumors.7 Thus in this study, REC8 was further compared between EBV-associated GC and EBV-negative GC. However, we found that REC8 expression was absent or downregulated in 9 of the 13 GC cell lines, including both EBV-positive and -negative cells. Inactivation of REC8 is directly associated with promoter methylation as shown by BGS and expressional restoration by demethylation agent treatment. Importantly, data from 223 GC samples of the TCGA study showed a significant negative correlation between methylation of the REC8 promoter and REC8 mRNA expression. We also detected silenced protein expression and significantly higher level of promoter methylation in gastric tumor samples as compared with normal gastric mucosa tissues in Chinese patients. These results confirm direct downregulation of REC8 by promoter methylation in GC. Notably, methylation level was significantly higher in 13 EBV-positive gastric tumors than in 18 EBV-negative gastric tumors from Chinese patients. Linear regression analysis in these samples further showed that EBV infection status was the only factor associated with a higher REC8 methylation level (Table 1).
We further enlarged the sample size of Chinese patients with gastric tumors for methylation-level quantification to investigate the clinical implication of REC8 promoter methylation. In a cohort of 191 GCs, regardless of EBV status, 94.2% (180 of 191) cases showed a methylation level >33.4%, the best cutoff value to discriminate gastric tumor from normal mucosa (Figure 3a). Using 33.4% as a cut point will group 94.2% (180 of 191) GC cases as ‘methylated' and the other 5.8% (11 of 191) cases as ‘unmethylated'. It would be meaningless to analyze the consequences of methylation of the REC8 promoter by comparing the 180 methylated cases with only 11 unmethylated cases. Therefore, we classified these cases into groups of ‘high methylation' and ‘low methylation' using 55% cutoff value by survival significance analysis using the tool, Cutoff Finder. Although there was no correlation between the REC8 low/high methylation and clinicopathological features, such as age, gender, H. pylori infection status, Lauren type, differentiation and TNM stage, multivariate Cox regression analysis revealed high methylation of REC8 promoter to be an independent predictor of poor survival in GC patients (Table 3).
We quantitated the methylation level of the REC8 promoter by direct Sanger sequencing of the PCR amplicons. As reviewed by Mikeska et al.,18 when heterogeneous methylation comprising multiple alleles with varied DNA methylation patterns (epialleles) is present, direct bisulfite sequencing of PCR products could not provide detailed information to characterize the heterogeneity of a sample. Our method assessed only the average methylation level of each CpG site within the PCR target region. The methylation status of the REC8 promoter, either heterogeneously methylated or comprising of a simple mixture of fully methylated and unmethylated alleles in gastric tumors, may not influence its value as a cancer biomarker. Poor peak quality with an increasing nucleotide number may happen when direct bisulfite sequencing is applied, especially for partially methylated samples.18 More convenient methods for methylation quantitation, such as bisulfite pyrosequencing and digital PCR approaches, should be developed to better quantitate the promoter methylation of REC8 for clinical implementation.
We then performed both gain- and loss-of-function experiments to investigate the role of REC8 in GC. Expression of REC8 exhibited marked growth-suppressing effect in both EBV-positive and -negative GC cells, whereas knockdown of REC8 significantly induced cell growth by increasing cell viability and clonogenicity. The growth-inhibitory effect of REC8 was further revealed to be related to the induction of apoptosis and inhibition of cell cycle progression by flow cytometry. The increased apoptosis induced by REC8 was further revealed by upregulation of the activated caspase-7 to stimulate the proteolytic cleavage of PARP for apoptosis initiation. Cell cycle analysis revealed that REC8 expression inhibited G1–S transition, with a significant increase of cells in G1 phase and decrease in S phase. The inhibition of cell cycle progression by REC8 was further demonstrated by the downregulated G1–S transition promoter (cyclin D1) and proliferation marker (PCNA) and the upregulated G1 gatekeeper (p27). Moreover, REC8 was observed to inhibit cell migration by induction of the cell–cell adhesion molecule, E-cadherin. With the similar functional results found in EBV-positive and -negative cell lines, we infer that the silencing of REC8 by promoter methylation has a similar role during gastric carcinogenesis in both EBV-positive and -negative subtypes. Although EBV infection is one of the multiple factors contributing to REC8 promoter methylation, the significantly higher methylation level in EBV-associated GC as compared with EBV-negative GCs might lead to new insights into guiding the management of EBV-associated GC.
We further elucidated the molecular basis of the tumor-suppressive effect of REC8 using cancer pathway cDNA microarray. We showed that REC8 exerted its antigrowth effect by inhibiting the cell proliferation regulators (G6PD and SLC2A1) and apoptosis inhibitor (NOL3), while inducing the expression of apoptosis regulator (GADD45G) and tumor suppressors (PinX1, IGFBP3 and ETS2). High levels of G6PD correlate with breast cancer metastasis and contribute to in vivo tumor growth.19 The glucose transporter, SLC2A1, has a vital role in glucose supply to cells, and PKA and cAMP stimulate cell proliferation by elevating SLC2A1 expression.20 The nucleolar protein, NOL3, is an endogenous inhibitor of apoptosis and promotes breast tumorigenesis and metastasis.21 GADD45G inhibits cell growth and induces apoptosis.22 PinX1 acts as a putative tumor suppressor by inhibiting telomerase.23 IGFBP3 impedes aggressive growth of pediatric liver cancer.24 A tumor-suppressor role has also been revealed for ETS2 in human non-small cell lung cancer.25 Moreover, REC8 caused cell cycle arrest at G1–S transition by inhibiting MCM2 and increasing PinX1. MCM2 is a cell cycle regulator promoting cell transmission from G1 to S phase.26 PinX1 has also been reported to inhibit G1–S transition and cell proliferation through the p16/cyclin D1 pathway in urothelial carcinoma of the bladder.23
The antimigration function of REC8 may be mediated by inhibiting epithelial–mesenchymal transition promoters SNAI1 and SNAI2, which are involved in generating de-differentiated cells, and inducing the migration inhibitor LDHA. SNAI1 and SNAI2 are zinc finger transcriptional repressors that have been reported to contribute to the aggressiveness of prostate cancer27 and invasiveness of malignant breast cancer,28 respectively. LDHA functions in anaerobic glycolysis. Attenuation of LDHA expression in cancer cells affects cytoskeletal structure and cell migration.29 Therefore, REC8 has a tumor-suppressive function by modulating the expression of important genes involved in multiple cellular processes, including proliferation, cell cycle, apoptosis, migration and differentiation.
In conclusion, we have identified that REC8 is a novel gene that is silenced by promoter hypermethylation in GC, especially the EBV subtype. REC8 has an important tumor-suppressive role in gastric carcinogenesis by modulating the important effectors involved in the regulation of cell proliferation, apoptosis, cell cycle and migration. More importantly, a high level of promoter methylation of REC8 is an independent risk factor for poor prognosis in GC patients. Epigenetic silencing of REC8 may contribute to the pathogenesis of GC.
Materials and methods
Cancer cell lines and culture condition
GC cell lines (AGS, NCI-N87, KatoIII) were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). AGS-EBV, an EBV-infected GC cell line,30 was a gift from Dr Shannon C Kenney (Department of Oncology and Medicine, McArdle Laboratory for Cancer Research at the University of Wisconsin, Madison, WI, USA). MKN28, MKN45, SNU16, SNU620, SNU638 and SNU719 cell lines were obtained from the Korean Cell Line Bank (Seoul, Korea). YCCEL1 was a gift from Sun Young Rha at Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea. BGC823, MGC803 and the immortalized normal human gastric epithelial cell line GES-1 were gifts from Oncology Hospital, Beijing University, Beijing, China. Cells were cultured in RPMI 1640, Dulbecco's modified Eagle's medium or McCoy medium (Gibco BRL, Rockville, MD, USA) supplemented with 10% fetal bovine serum (Gibco BRL).
Human samples
GC tissue samples were collected from the First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China from 1999 to 2006 and from the Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, Hong Kong, from 2005 to 2013. The presence of EBV was determined by detection of EBER carried out as previously reported by us.31 H. pylori status was assessed by examining the Gram-negative curved bacilli on histology.4 All patients gave informed consent for participation in this study. This study was approved by both the Clinical Research Ethics Committee of Sun Yat-sen University and the ethics committee of the Chinese University of Hong Kong.
Bisulfite genomic sequencing
BGS and combined bisulfite restriction analysis were performed to evaluate methylation status. PCR amplification was performed with 2 μl bisulfite-converted DNA. Direct Sanger sequencing of the PCR products was used to evaluate methylation levels at multiple CpG sites. The proportion of methylation was calculated as the peak ratio of cytosine to the sum of cytosine and thymine at each site. Primers used are listed in our previous study.7
Demethylation with 5-Aza agent treatment
GC cells were treated with 2 μm DNA demethylation agent 5-Aza (Sigma-Aldrich, St Louis, MO, USA) for 5 days, and the medium was refreshed every day.
Immunohistochemistry
Paired primary tumor and adjacent non-tumor samples were obtained from 12 GC patients after surgical resection. Tissue types (tumor or normal) were assessed by histological staining. The remaining tissue specimens were fixed in 10% formalin and embedded in paraffin. Immunohistochemistry was performed on 5-μm paraffin sections using anti-REC8 antibodies (Abcam, Cambridge, UK) as previously described.5, 32 REC8 staining in the nucleus and cytoplasm was evaluated by scanning the whole section and counting >1000 representative cells.
RNA extraction, RT–PCR and quantitative PCR
Total RNA was extracted from cell pellets or tissues using Qiazol reagent (Qiagen, Valencia, CA, USA), and cDNA was synthesized using Transcriptor Reverse Transcriptase (Roche, Indianapolis, IN, USA). RT–PCR was performed using the Go-Taq DNA polymerase (Promega, Madison, MI, USA). Quantitative PCR was performed using SYBR Green master mixture on HT7900 system (Applied Biosystems, Foster City, CA, USA). Primers used are listed in our previous study.7
Correlation between REC8 promoter methylation and mRNA expression
Methylation (HM450) and mRNA expression (RNA Seq V2 RSEM) data from TCGA Stomach Adenocarcinoma (Provisional) study were retrieved at cBioPortal (http://www.cbioportal.org/public-portal/index.do).33, 34 In total, 223 TCGA gastric samples (follow-up time=1.18 (0.46–8.92) months, median (interquartile range)) with both methylation and mRNA expression data were included in this study. Linear regression was performed on Log10 mRNA levels and methylation levels.
Western blotting
Total protein was extracted and protein concentration was then measured by the DC protein assay method of Bradford (Bio-Rad, Hercules, CA, USA). Proteins were separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes (GE Healthcare, Piscataway, NJ, USA). Blots were immunostained with primary antibodies overnight and then with secondary antibody at room temperature for 1 h. Proteins of interest were visualized using ECL Plus Western blotting Detection Reagents (GE Healthcare). Antibodies against GAPDH (glyceraldehyde 3-phosphate dehydrogenase; sc-25778) and E-cadherin (sc-21791) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against Cyclin D1 (no. 2978), p27 (no. 3686), Cleaved Caspase-7 (no. 9491) and Cleaved PARP (no. 5625) were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibody against PCNA (ab29) was purchased from Abcam.
Construction of vector and ectopic expression of REC8
Full length of the open reading frame of human REC8 was generated by RT–PCR. The PCR products were confirmed by direct DNA sequencing and cloned into a mammalian expression vector, pcDNA3.1 (Invitrogen, Carlsbad, CA, USA). The sequence of the construct was further confirmed by sequencing. The resulting expression vectors were transfected into cells with low expression of REC8 (AGS-EBV and BGC823) using Lipofectamine 2000 (Invitrogen). Stably transfected cells were established under selection with neomycin (G418) (Invitrogen). Empty vectors were used as control for transfection.
Knockdown of REC8
A set of vectors carrying shRNAs against REC8 was purchased from Origene (Rockville, MD, USA). The REC8-expressing cell lines GES-1 and AGS were transfected with vectors carrying scrambled sequence (sh-scrambled) as negative control. Knockdown efficiency was evaluated 48 h after transfection by western blotting. Puromycin (Invitrogen) was used to establish stable knockdown cells for colony formation and cell viability assays.
Cell viability assay
Cell viability was assessed by the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay (Promega).5 The experiments were performed three times independently.
Colony-formation assay
Colony formation was performed as described previously.5 The experiments were performed three times independently.
Cell cycle analysis
AGS-EBV and BGC823 cells transfected with REC8 expression vector or empty vector were fixed in 70% ethanol–phosphate-buffered saline for 24 h and labeled with 50 μg/ml propidium iodide (BD Biosciences, Franklin Lakes, NJ, USA). The cells were sorted by Fluorescence-Activated Cell Sorting Calibur Flow Cytometer (BD Biosciences), and cell cycle distributions were analyzed using the ModFitLT software (BD Biosciences).
Apoptosis
Cell apoptosis was determined by staining cells with Annexin V and 7-AAD (BD Biosciences) with subsequent flow cytometric analysis. Cell populations were counted as viable (Annexin V-negative, 7-AAD-negative), early apoptotic (Annexin V-positive, 7-AAD-negative), late apoptotic (Annexin V-positive, 7-AAD-positive) or necrotic (Annexin V-negative, 7-AAD-positive).
Cell migration assay
Cell migration was evaluated using wound-healing assay for three independent experiments as previously described.5
In vivo tumorigenicity
BGC823 cells (1 × 107 cells in 0.1 ml phosphate-buffered saline) stably transfected with REC8 expression vector or empty vector were injected subcutaneously into the dorsal flank of 4-week-old male Balb/c nude mice (n=5/group). Tumor diameter was measured every 2 days for 2 weeks. Animal experimental procedures were approved by the Animal Ethics Committee of the Chinese University of Hong Kong.
Human cancer pathway finder RT2 profiler PCR array
Gene expression profiles of GC cell lines AGS-EBV transiently transfected with REC8 or control plasmid were analyzed by the Human Cancer PathwayFinder RT2 Profiler PCR Array (Qiagen), which contained 84 well-characterized genes with representative roles in tumorigenesis.12
Statistical analysis
The results were expressed as mean±s.d. or median (interquartile range). Mann–Whitney U-test was performed to compare the variables of the two sample groups. ROC curve was used to estimate the cutoff value of the methylation percentage. Methylation cutoff value was analyzed by survival significance analysis using the tool Cutoff Finder (http://molpath.charite.de/cutoff/).17 The difference in tumor growth rate between the two groups of nude mice was determined by repeated-measures analysis of variance. All statistical tests were performed using Graphpad Prism 5.0 (Graphpad Software Inc., San Diego, CA, USA) or the SPSS program (version 17.0; SPSS, Chicago, IL, USA). Value of P<0.05 was taken as statistical significance.
Acknowledgments
This project was supported by research funds of HMRF (11100022), RGC-GRF Hong Kong (766613), National Natural Science Foundation of China (81272304), China 863 Program (2012AA02A504), Shenzhen Municipal Science and Technology R & D fund (JCYJ20130401151108652) and Shenzhen Virtual University Park Support Scheme to CUHK Shenzhen Research Institute. The results shown here are in part based upon data generated by the TCGA Research Network: http://cancergenome.nih.gov/.
Author contributions
JW, KW, JG, JZ and YZ performed the experiments; JY and QL analyzed data and drafted the paper; PWC and EKN provided clinical samples; JJS commented on the study; JY designed, supervised study and revised the paper.
Glossary
- 7-AAD
7-aminoactinomycin D
- BGS
bisulfite genomic sequencing
- cDNA
complementary DNA
- EBV
Epstein–Barr virus
- GC
gastric cancer
- PARP
poly (ADP-ribose) polymerase
- RT–PCR
reverse transcription PCR.
The authors declare no conflict of interest.
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- Wang S, Cheng Y, Du W, Lu L, Zhou L, Wang H et al. Zinc-finger protein 545 is a novel tumour suppressor that acts by inhibiting ribosomal RNA transcription in gastric cancer. Gut 2013; 62: 833–841. [DOI] [PubMed] [Google Scholar]
- Xu L, Li X, Chu ES, Zhao G, Go MY, Tao Q et al. Epigenetic inactivation of BCL6B, a novel functional tumour suppressor for gastric cancer, is associated with poor survival. Gut 2012; 61: 977–985. [DOI] [PubMed] [Google Scholar]
- Yu J, Cheng YY, Tao Q, Cheung KF, Lam CN, Geng H et al. Methylation of protocadherin 10, a novel tumor suppressor, is associated with poor prognosis in patients with gastric cancer. Gastroenterology 2009; 136: 640–651 e641. [DOI] [PubMed] [Google Scholar]
- Yu J, Liang QY, Wang J, Cheng Y, Wang S, Poon TC et al. Zinc-finger protein 331, a novel putative tumor suppressor, suppresses growth and invasiveness of gastric cancer. Oncogene 2013; 32: 307–317. [DOI] [PubMed] [Google Scholar]
- Camargo MC, Kim WH, Chiaravalli AM, Kim KM, Corvalan AH, Matsuo K et al. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: an international pooled analysis. Gut 2014; 63: 236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Liang Q, Cheung KF, Kang W, Lung RW, Tong JH et al. Genome-wide identification of Epstein-Barr virus-driven promoter methylation profiles of human genes in gastric cancer cells. Cancer 2013; 119: 304–312. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014; 513: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Q, Yao X, Tang S, Zhang J, Yau TO, Li X et al. Integrative identification of Epstein-Barr virus-associated mutations and epigenetic alterations in gastric cancer. Gastroenterology 2014; 147: 1350–1362 e1354. [DOI] [PubMed] [Google Scholar]
- Hino R, Uozaki H, Murakami N, Ushiku T, Shinozaki A, Ishikawa S et al. Activation of DNA methyltransferase 1 by EBV latent membrane protein 2A leads to promoter hypermethylation of PTEN gene in gastric carcinoma. Cancer Res 2009; 69: 2766–2774. [DOI] [PubMed] [Google Scholar]
- Sudo M, Chong JM, Sakuma K, Ushiku T, Uozaki H, Nagai H et al. Promoter hypermethylation of E-cadherin and its abnormal expression in Epstein-Barr virus-associated gastric carcinoma. Int J Cancer 2004; 109: 194–199. [DOI] [PubMed] [Google Scholar]
- Zhao J, Liang Q, Cheung KF, Kang W, Dong Y, Lung RW et al. Somatostatin receptor 1, a novel EBV-associated CpG hypermethylated gene, contributes to the pathogenesis of EBV-associated gastric cancer. Br J Cancer 2013; 108: 2557–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K. Disseminating the genome: joining, resolving, and separating sister chromatids during mitosis and meiosis. Annu Rev Genet 2001; 35: 673–745. [DOI] [PubMed] [Google Scholar]
- Liu DX, Shen XP, Zhu GW, Xing MZ. REC8 is a novel tumor suppressor gene epigenetically robustly targeted by the PI3K pathway in thyroid cancer. Oncotarget 2015; 6: 39211–39224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wockner LF, Morris CP, Noble EP, Lawford BR, Whitehall VLJ, Young RM et al. Brain-specific epigenetic markers of schizophrenia. Transl Psychiatry 2015; 5: e680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y, Sawaki A, Ito S, Nishida T, Takahashi T, Toyota M et al. Aberrant DNA methylation associated with aggressiveness of gastrointestinal stromal tumour. Gut 2012; 61: 392–401. [DOI] [PubMed] [Google Scholar]
- Budczies J, Klauschen F, Sinn BV, Gyorffy B, Schmitt WD, Darb-Esfahani S et al. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One 2012; 7: e51862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikeska T, Candiloro IL, Dobrovic A. The implications of heterogeneous DNA methylation for the accurate quantification of methylation. Epigenomics 2010; 2: 561–573. [DOI] [PubMed] [Google Scholar]
- Du W, Jiang P, Mancuso A, Stonestrom A, Brewer MD, Minn AJ et al. TAp73 enhances the pentose phosphate pathway and supports cell proliferation. Nat Cell Biol 2013; 15: 991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MO, Lee YJ, Park JH, Ryu JM, Yun SP, Han HJ. PKA and cAMP stimulate proliferation of mouse embryonic stem cells by elevating GLUT1 expression mediated by the NF-kappaB and CREB/CBP signaling pathways. Biochim Biophys Acta 2012; 1820: 1636–1646. [DOI] [PubMed] [Google Scholar]
- Medina-Ramirez CM, Goswami S, Smirnova T, Bamira D, Benson B, Ferrick N et al. Apoptosis inhibitor ARC promotes breast tumorigenesis, metastasis, and chemoresistance. Cancer Res 2011; 71: 7705–7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying J, Srivastava G, Hsieh WS, Gao Z, Murray P, Liao SK et al. The stress-responsive gene GADD45G is a functional tumor suppressor, with its response to environmental stresses frequently disrupted epigenetically in multiple tumors. Clin Cancer Res 2005; 11: 6442–6449. [DOI] [PubMed] [Google Scholar]
- Liu JY, Qian D, He LR, Li YH, Liao YJ, Mai SJ et al. PinX1 suppresses bladder urothelial carcinoma cell proliferation via the inhibition of telomerase activity and p16/cyclin D1 pathway. Mol Cancer 2013; 12: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regel I, Eichenmuller M, Joppien S, Liebl J, Haberle B, Muller-Hocker J et al. IGFBP3 impedes aggressive growth of pediatric liver cancer and is epigenetically silenced in vascular invasive and metastatic tumors. Mol Cancer 2012; 11: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbout M, Garcia MM, Fujimoto J, Liu DD, Woods D, Chow CW et al. ETS2 mediated tumor suppressive function and MET oncogene inhibition in human non-small cell lung cancer. Clin Cancer Res 2013; 19: 3383–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt SC, Bailey KJ, Freeland A. Reduced Mcm2 expression results in severe stem/progenitor cell deficiency and cancer. Stem Cells 2007; 25: 3121–3132. [DOI] [PubMed] [Google Scholar]
- Deep G, Jain AK, Ramteke A, Ting H, Vijendra KC, Gangar SC et al. SNAI1 is critical for the aggressiveness of prostate cancer cells with low E-cadherin. Mol Cancer 2014; 13: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhang B, Li W, Fu L, Zhu Z, Dong JT. Epigenetic silencing of miR-203 upregulates SNAI2 and contributes to the invasiveness of malignant breast cancer cells. Genes Cancer 2011; 2: 782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arseneault R, Chien A, Newington JT, Rappon T, Harris R, Cumming RC. Attenuation of LDHA expression in cancer cells leads to redox-dependent alterations in cytoskeletal structure and cell migration. Cancer Lett 2013; 338: 255–266. [DOI] [PubMed] [Google Scholar]
- Feng WH, Kraus RJ, Dickerson SJ, Lim HJ, Jones RJ, Yu X et al. ZEB1 and c-Jun levels contribute to the establishment of highly lytic Epstein-Barr virus infection in gastric AGS cells. J Virol 2007; 81: 10113–10122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Jin H, Cheung KF, Tong JH, Zhang S, Go MY et al. Zinc finger E-box binding factor 1 plays a central role in regulating Epstein-Barr virus (EBV) latent-lytic switch and acts as a therapeutic target in EBV-associated gastric cancer. Cancer 2012; 118: 924–936. [DOI] [PubMed] [Google Scholar]
- Yu J, Leung WK, Ebert MP, Ng EK, Go MY, Wang HB et al. Increased expression of survivin in gastric cancer patients and in first degree relatives. Br J Cancer 2002; 87: 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012; 2: 401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013; 6: pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]