Abstract
Recent studies in animal systems have shown that NO can interact with fatty acids to generate nitro-fatty acids (NO2-FAs). They are the product of the reaction between reactive nitrogen species and unsaturated fatty acids, and are considered novel mediators of cell signaling based mainly on a proven anti-inflammatory response. Although these signaling mediators have been described widely in animal systems, NO2-FAs have scarcely been studied in plants. Preliminary data have revealed the endogenous presence of free and protein-adducted NO2-FAs in extra-virgin olive oil (EVOO), which appear to be contributing to the cardiovascular benefits associated with the Mediterranean diet. Importantly, new findings have displayed the endogenous occurrence of nitro-linolenic acid (NO2-Ln) in the model plant Arabidopsis thaliana and the modulation of NO2-Ln levels throughout this plant's development. Furthermore, a transcriptomic analysis by RNA-seq technology established a clear signaling role for this molecule, demonstrating that NO2-Ln was involved in plant-defense response against different abiotic-stress conditions, mainly by inducing the chaperone network and supporting a conserved mechanism of action in both animal and plant defense processes. Thus, NO2-Ln levels significantly rose under several abiotic-stress conditions, highlighting the strong signaling role of these molecules in the plant-protection mechanism. Finally, the potential of NO2-Ln as a NO donor has recently been described both in vitro and in vivo. Jointly, this ability gives NO2-Ln the potential to act as a signaling molecule by the direct release of NO, due to its capacity to induce different changes mediated by NO or NO-related molecules such as nitration and S-nitrosylation, or by the electrophilic capacity of these molecules through a nitroalkylation mechanism. Here, we describe the current state of the art regarding the advances performed in the field of NO2-FAs in plants and their implication in plant physiology.
Keywords: Nitro-fatty acids, Nitro-linolenic acid, Signaling molecule, Antioxidant response, Oxidative stress, Nitric oxide donor, Defense response, Plants
Graphical abstract
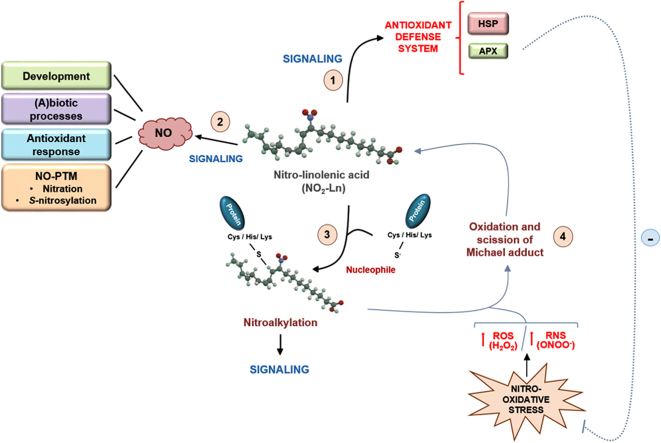
Nitro-fatty acid signaling in plants. Pathway 1 indicates that NO2-Ln is able to induce a defense mechanism through the induction of the chaperone network and the increase in the expression of some antioxidant enzymes such as APX. This latter may be involved in the alleviation of the oxidative stress generated by the overproduction of ROS such as H2O2. Furthermore, NO2-Ln is a NO donor being therefore implicated in the wide range of actions in which this molecule is involved (pathway 2). The electrophilic ability of this nitro-fatty acid could contribute to the signaling actions involving NO2-FAs (pathway 3) and, under nitro-oxidative stress conditions, the oxidation of Michael adducts may occur with subsequent NO2-FA release (pathway 4) and the observed antioxidant properties of these molecules. NO: nitric oxide; HSP: heat shock protein; APX: ascorbate peroxidase.
Highlights
-
•
Nitro-Linolenic acid is an endogenous molecule whose levels are modulated throughout Arabidopsis development.
-
•
Nitro-Linolenic acid induces the molecular chaperone network in Arabidopsis.
-
•
Nitro-fatty acids are able to act as a signaling mediators in the plant-defense mechanism in abiotic and oxidative stress situations, setting up a defense response against cell damage.
-
•
Nitro-fatty acids have been demonstrated to be in vitro and in vivo NO donors.
-
•
The nitro-fatty acid detection in other plant species highlight a ubiquitous distribution of nitro-fatty acids in plant kingdom and the potential signaling actions of these molecules in plant systems.
1. Introduction
Electrophilic nitro-fatty acids (NO2-FAs) are endogenously formed by redox reactions of nitric oxide (·NO) and NO-derived molecules that promote the generation of nitrogen dioxide (·NO2); which in turn nitrates unsaturated fatty acids [1], [2], [3]. The first report describing the endogenous occurrence of lipid nitrated derivatives was defined both in normolipidemic and hyperlipidemic donors [4]. Numerous studies have since reported a selective nitration of fatty acids under physiological conditions, highlighting the greater susceptibility to nitration of conjugated fatty acids than methyl-interrupted dienes or polyenes in animal systems [5]. Most of these analyses have shown that these molecules trigger pleiotropic signaling actions including a remarkable anti-inflammatory and antioxidant response [6], [7], [8], [9]. A measurable increase in the levels of NO2-FAs has been shown under different pathological conditions such as ischemia reperfusion injury or after LPS injection in the peritoneum [1], [10].
Currently, the formation mechanisms of NO2-FAs remain unknown although different ways have been proposed, such as by ·NO2, peroxynitrite (ONOO-) or protonation of nitrite (NO2-) yielding nitrous acid (HNO2) and subsequent nitrating and nitrosating species [11]. By contrast, several ways of inactivating nitro-fatty acid signaling have recently been described. In this regard, the NADPH-dependent enzyme prostaglandin reductase-1 (PGR-1) is involved in the inactivation of NO2-FAs in the liver through a saturation mechanism [12]. Moreover, conjugation of these molecules to reduced glutathione (GSH) and subsequent β-oxidation and excretion through urine has also been noted as a mechanism of inactivation of NO2-FAs [13]. Furthermore, due to the hydrophobic nature of these molecules, cell membranes and/ or lipoproteins may constitute a natural source of NO2-FAs [14], [15]. Based on the electrophilic capacity of nitro-fatty acids, an important part of the endogenous content of these molecules could also be found adducted with different nucleophiles, mainly with proteins [16]. In fact, the intravenous injection of nitro-oleic acid (NO2-OA) in mice has shown that this NO2-FA is rapidly adducted to plasma thiol-containing proteins and glutathione, and this being only a small portion of free NO2-OA in plasma [1]. Furthermore, it has been recently described the release of NO2-FAs from Cys-adducted nitroalkenes under nitro-oxidative stress situations [17]. Representative ROS and RNS, such as hydrogen peroxide (H2O2) and ONOO- respectively, are able to initiate the release of free nitroalkenes from protein-adducted NO2-FAs. These nitro-oxidative conditions promote the formation of a β-nitrosulfoxide intermediate and consequently releasing free NO2-FAs. This behavior may have relevance under several stressful situations both in animal and plant systems such as inflammation or under different adverse environmental conditions in which an increase of ROS and RNS has been noted [18], [19], [20], [21], [22]. Actually, the anti-inflammatory and antioxidant signaling properties of these molecules have been attributed to free NO2-FAs [23], [24], [25], [26], [27].
The beneficial properties of NO2-FAs described above have been attributed mainly to the electrophilic capacity of the β-carbon of the reactive nitroalkenyl substituent from NO2-Fas, which readily undergoes a reversible Michael addition [2], [28]. This reaction is known specifically as nitroalkylation and occurs primarily with functionally significant Cys and His residues on transcription-regulating proteins and enzymes (Fig. 1) [2], [16], [28]. Through this post-translational alteration, NO2-FAs can reportedly exert pleiotropic signaling actions, particularly an anti-inflammatory and antioxidant response [2], [29]. In this respect, electrophilic nitroalkenes such as nitro-oleic (NO2-OA) or nitro-linoleic (NO2-LA) acids have been identified as antagonists of the nuclear lipid receptor peroxisome proliferator-activated receptor-γ (PPARγ) [30], [31], the Keap1-Nrf2 signaling pathway [6], [32], and the heat-shock response [26]. Furthermore, the antagonistic properties of NO2-FAs on NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) also promote a strong anti-inflammatory response [23].
Fig. 1.
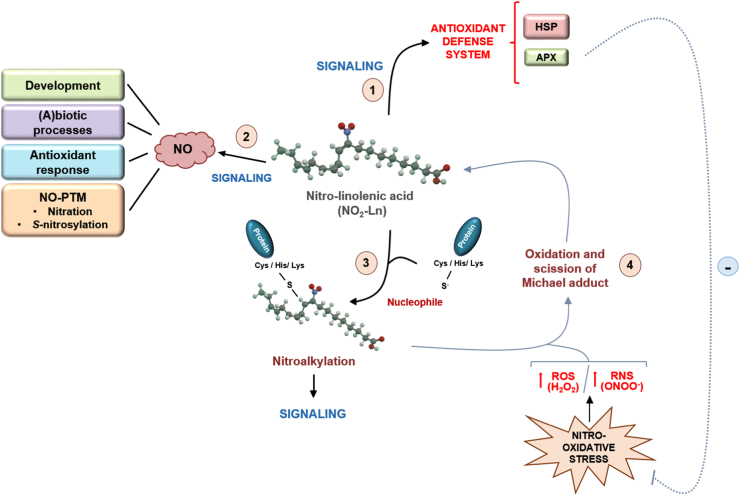
Schematic model of NO2-Ln signaling. Pathway 1 indicates that NO2-Ln is able to induce a defense mechanism through the induction of the chaperone network and the increase in the expression of ascorbate peroxidase enzyme. This latter may be involved in the alleviation of the oxidative stress generated by the overproduction of ROS such as H2O2. Furthermore, NO2-Ln is a NO donor being therefore implicated in the wide range of actions in which this molecule is involved (pathway 2). The electrophilic ability of this nitro-fatty acid could contribute to the signaling actions involving NO2-FAs (pathway 3) and, under nitro-oxidative stress conditions, the oxidation of Michael adducts may occur with subsequent nitroalkene release (pathway 4) and the observed antioxidant properties of these molecules. NO: nitric oxide; HSP: heat shock protein; APX: ascorbate peroxidase.
On the other hand, the ability of NO2-FAs to release NO has been thoroughly described [33], [34], [35], but the precise mechanism of NO release has not yet been identified. Nevertheless, [34] postulated that NO released from NO2-LA may be based on a modified Nef reaction [34]. In this sense, deprotonation of the carbon γ to the nitro group leads to the formation of an unstable nitroso intermediate that rapidly degrades to release either HNO, the predicted Nef reaction product, or ·NO, a product considered likely due to the weak C-N bond present on the hydroxyl-nitroso intermediate. An alternative mechanism to the modified Nef reaction was also hypothesized based on a rearrangement of the nitroalkene to a nitrite ester, followed by N-C bond hemolysis to form ·NO and a lipid radical [36]. The release of NO from NO2-FAs has been considered a very minor component of the diverse biological actions of these molecules inasmuch as the β-carbon adjacent to the nitro group is strongly electrophilic and reacts covalently with protein and thiols [16], [37], [38]. However, NO release from NO2-LA mediates the S-nitrosylation of the pro-inflammatory member CD40 with subsequent inactivation, thereby triggering an anti-inflammatory response [39]. Therefore, we cannot rule out that this NO release can have important consequences for key cellular targets.
Although the occurrence and the biological actions of NO2-FAs have been widely explored in animal systems, the study of these molecules in plant systems constitutes an interesting and emerging area. In this regard, the present review describes the current state of the art with respect to the advances in the study of NO2-FAs in plant systems and their implications in plant physiology.
2. Characterization of the endogenous occurrence of NO2-FAs in plant systems
2.1. Extra-virgin olive oil (EVOO) and olives
The Mediterranean diet constitutes a key source of unsaturated fatty acids with extra-virgin olive oil (EVOO) providing health benefits to humans. The Mediterranean diet is associated with different anti-inflammatory and anti-hypertensive effects leading to a reduced risk of cardiovascular morbidity and mortality [40], [41], [42]. Based on the previously reported anti-inflammatory properties attributed to NO2-FAs, an initial study analyzing the endogenous occurrence of these molecules was made in EVOO and fresh olives [43]. In this respect, the authors noted the presence of nitrated derivatives of conjugated linoleic acid (NO2-cLA) in EVOO, specifically of 9- and 12-NO2-cLA isomers. Moreover, the Mediterranean diet is also characterized by the high intake of leafy vegetables and cured meats, which are high in nitrates (NO3-) and nitrites (NO2-). NO3- can be reduced by bacteria in the saliva to NO2- and the latter can form nitrous acid (HNO2) in the stomach being thereby broken down to NO2. Furthermore, oils, fish, several seeds, and dairy products such as milk or cheese are rich sources of ω−3 and ω−6 fatty acids, including oleic, linoleic and linolenic acids. These factors together with acidic conditions of digestion may lead to the gastric generation of NO2-FAs. In this regard, the incubation of EVOO with gastric juice artificial and 15N-labelled sodium nitrite (Na[15N]O2) results in the detection of different NO2-FAs such as 9- and 10-NO2-OA; 9/10-, 12- and 13-NO2-LA and 8-, 9-, 11- and 12-NO2-cLA [43]. Finally, NO2-OA-cysteine adducts have been endogenously detected in acid-hydrolyzed proteins from the peel and mesocarp of freshly picked whole olives of different cultivars. For example, NO2-OA-cysteine levels were higher in Picual olive cultivars and more abundant in the peel from these olives [43], underscoring that EVOO and olives are both a source and metabolic reserve of NO2-FAs.
Importantly, the ex vivo detection of NO2-OA and NO2-LA in EVOO may be responsible in part for the numerous beneficial properties attributed to this important source of fatty acids in the Mediterranean diet. Furthermore, nitrated derivatives of oleic and linoleic acids not only inhibit leukocyte and platelet activation [44], vascular smooth-muscle proliferation [6], and lipopolysaccharide-stimulated macrophage cytokine secretion [23], but also activate PPARγ receptor [45] and induce endothelial heme oxygenase 1 expression [46]. These NO2-FAs also strongly modulate Nrf2/Keap1 [6], [23] and NF-κB-regulated inflammatory signaling [23], as previously mentioned. Therefore, the presence of nitrated derivatives of oleic, linoleic or conjugated linoleic acids may contribute to the cardiovascular benefits associated with the Mediterranean diet.
2.2. The model plant Arabidopsis thaliana
Previous findings on the endogenous occurrence of NO2-FAs in EVOO and olives prompted the analysis of the presence of these molecules in the model plant arabidopsis (Arabidopsis thaliana). The study was performed using mass spectrometric approaches in 9-d-old arabidopsis cell-suspension cultures (ACSC) and in 14-d-old seedlings [27]. Firstly, lipid composition analysis of 9-d-old ACSC and 14-d-old seedlings revealed that linolenic acid (18Δ3) was the most abundant unsaturated fatty acid in both samples followed by linoleic (18Δ2) and oleic (18Δ1) acids. This background motivated the study of the presence of nitrated derivatives of the most abundant unsaturated fatty acids, and therefore an analysis was made of the presence of NO2-OA, NO2-LA and nitro-linolenic acid (NO2-Ln). Synthesis of these different standards by a nitroselenation procedure made it possible to identify the only endogenous occurrence of NO2-Ln by comparing the retention time of the chromatographic peak of NO2-Ln standard to those observed in both 9-d-old ACSC and 14-d-old seedlings [27]. As confirmation that the peak detected corresponded to electrophilic NO2-Ln, a nitroalkylation reaction with β-mercaptoethanol (β-ME) of the lipid extract was carried out by seeking the covalent nitroalkylated adduct formed by the reaction of NO2-Ln and the thiol of β-ME. After incubation, the retention time of the peaks detected changed, confirming the electrophilic capacity of NO2-Ln and showing the endogenous presence of this nitro-fatty acid in arabidopsis. Furthermore, the NO2-Ln content was analyzed in both samples (Table 1) and the results showing 0.28±0.04 pmol/g FW in 9-d-old ACSC and 3.84±0.44 pmol/ g FW in 14-d-old seedlings [27]. These low levels were consistent with previous results found in the field of animal systems for other nitro-fatty acids such as NO2-OA [47] and NO2-LA suggesting these molecules could act as important signaling components of plant physiology. Moreover, the presence of other nitrated derivatives from oleic, linoleic and conjugated-linoleic acids was also analyzed. In all studied samples, the endogenous occurrence of nitro-oleic acid (MRM transition 326/279 m/z) was not observed due to no chromatographic peaks were detected with this MRM transition. Regarding the presence of nitro-linoleic or nitro-conjugated linoleic acids with MRM transition 324/277 m/z, a chromatographic peak with this MRM transition was observed. Nevertheless, the incubation of lipid extracts from all the analyzed samples with the electrophile β-ME did not make disappear those chromatographic peaks assuring they did not correspond to any of these NO2-FAs.
Table 1.
Endogenous detection of nitro-linolenic acid (NO2-Ln) in several plant species and in different subcellular fractions. ACSC, Arabidopsis cell suspension cultures.
| Plant species | Organ/Subcellular fraction | pmol NO2-Ln/ g FW |
|---|---|---|
| Arabidopsis thaliana | 14-d-old seedlings | 3.84 |
| 9-d-old ACSC | 0.28 | |
| Pea (Pisum sativum) | Roots | 0.072 |
| Leaves | 0.084 | |
| Mitochondria | ||
| Peroxisomes | 0.282 | |
| Rice (Oryza sativa) | Leaf | 0.748 |
2.3. Nitro-fatty acid detection in other plant species
Besides showing the endogenous presence of NO2-Ln in the arabidopsis, the detection of NO2-FAs in other plant species was also analyzed (Table 1). In line with the approaches followed in arabidopsis, nitrated derivatives from the most abundant unsaturated fatty acids were found. This analysis resulted in the endogenous detection of NO2-Ln in roots from pea (Pisum sativum) plants and in leaves from rice (Oryza sativa) plants, the levels of this nitro-fatty acid being 0.072 and 0.748 pmol/ g FW, respectively (Table 1). Mitochondrial and peroxisomal fractions from pea plants were also subjected to the analysis of the different NO2-FAs, revealing NO2-Ln in both samples. The content of this molecule was about 0.084 and 0.282 pmol/g FW, in the mitochondrial and peroxisomal fractions, respectively (Table 1). In all analyzed samples was only possible to detect the endogenous occurrence of NO2-Ln, but not other nitrated derivatives from major fatty acids such as NO2-OA or NO2-LA, suggesting the higher susceptibility of linolenic acid to be endogenously nitrated and the relevance of this NO2-FA in plant systems. All these results also highlight a ubiquitous distribution of NO2-FAs in plant kingdom and the potential signaling actions of these molecules in plant systems.
3. Involvement of nitro-fatty acids at development and under abiotic stress
In light of the outcomes of the A. thaliana analyses, the study concerning the levels of NO2-Ln was extended throughout plant development and under different stress conditions, specifically abiotic stress. Furthermore, based on the low levels of NO2-Ln in plant systems, a transcriptomic analysis with RNA-seq technology helped to elucidate the potential signaling role of this molecule in plant physiology.
3.1. Modulation of NO2-Ln content throughout the development of Arabidopsis
As mentioned above, the endogenous occurrence of NO2-Ln was observed in 9-d-old ACSC and in 14-d-old seedlings. These results prompted an analysis of the content of NO2-Ln throughout the developmental process of arabidopsis. In this sense, different arabidopsis plant materials such as seeds, 14-d-old seedlings, and leaves from 30- and 45-d-old plants, with clear symptoms of senescence the latter, were used for this study. Higher levels of NO2-Ln were found at the beginning of the development, specifically in seeds (11.18±1.68 pmol/g FW) and in 14-d-old seedlings (3.84±0.44 pmol/g FW). Nevertheless, this content significantly declined with the progress of plant development and with senescence in leaves from 30- (0.36±0.04 pmol/g FW) and 45-d-old plants (0.54±0.06 pmol/g FW) [27]. Nitro-fatty acids have been demonstrated to be in vitro and in vivo NO donors [33], [34], [35], [48]. NO has a major role at the beginning of plant development because this molecule triggers seed germination [49], among other key processes in plant physiology. The higher content of NO2-Ln detected in seeds may imply that this nitro-fatty acid acts as a NO donor at this stage and therefore favors germination and the onset of vegetative development. In this respect, it has been recently shown that S-nitrosylation of the ABA-related transcription factor, ABI5 promotes the interaction with CUL4-based and KEG E3 ligases, which are subsequently degraded by the proteasome, promoting seed germination [50]. Therefore, it cannot be ruled out that NO2-Ln could release NO in seeds and mediate a S-nitrosylation mechanism of key transcription factors such as ABI5, thereby stimulating seed germination.
3.2. Raising of NO2-Ln levels under abiotic-stress situations
Besides the involvement of NO and other NO-related molecules in several physiological processes such as germination, growth, development, and senescence [22], [49], [51], [52], [53], [54], [55], [56], this gaseous molecule is also involved in the response to several (a)biotic stress conditions such as pathogen infection, symbiotic interactions, mechanical wounding, salinity, UV-radiation, and heavy-metal stress [21], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67]. In relation to this implication, the levels of NO2-Ln were analyzed under different abiotic-stress conditions. With 9-d-old ACSC exposure to 100-mM NaCl, a significant rise of NO2-Ln content was observed after 5 min of treatment (0.96±0.12 pmol/g FW) compared to steady-state levels (0.28±0.04 pmol/ g FW), which tripled the levels of this nitro-fatty acid. Nevertheless, after 30 min of salt stress the levels of NO2-Ln fell [27]. Moreover, in 14-d-old seedlings subjected to mechanical wounding, heavy-metal and low-temperature stress, a noteworthy rise in NO2-Ln levels was recorded, values being 7.46±1.20, 6.62±0.98 and 5.75±0.79 pmol/g FW, respectively, with regard to control seedlings (3.84±0.44 pmol/g FW) [27]. These results indicate the involvement of NO2-Ln in the response to different abiotic-stress situations.
4. Signaling role of NO2-Ln in plant physiology
Because the NO2-Ln content was modulated throughout plant's development and under different stressful situations together with the low observed levels of this molecule in plants systems, a transcriptomic analysis was performed to define the potential signaling actions of this molecule in plant physiology. Furthermore, based on the implications of NO2-Ln in a wide range of actions, the ability to release NO both in vitro and in vivo was also assessed.
4.1. Transcriptomic analysis of NO2-Ln-responsive genes
To establish the potential signaling mechanisms in which NO2-Ln was involved, a transcriptomic analysis by RNA-seq technology was performed by analyzing 10 µM and 100 µM concentrations of this nitro-fatty acid in 9-d-old ACSC [27]. The use of these concentrations of NO2-Ln indicated a clear signaling response of this molecule in plant physiology, in agreement with concentrations used in previous studies in animal systems [13], [47]. Importantly, the application of NO2-Ln to ACSC prompted a dose-dependence response ranging from 1 µM to 100 µM NO2-Ln which was shown by qRT-PCR and by DNAstar software [27], this having been previously described for NO2-OA or NO2-cLA in animal systems [5], [26].
Firstly, with the use of the Blast2GO suite, a Gene Ontology term-enrichment analysis of NO2-Ln-responsive genes showed that over-expressed genes were related to stress response, but specifically with the response to abiotic stress and to oxidative stress-related processes. By contrast, down-regulated genes were involved in some biological processes and especially with the biosynthesis of several cell compounds [27]. Therefore, these results suggest a close relationship between NO2-Ln and the involvement in different abiotic-stress conditions. A subset of genes modulated by NO2-Ln in ACSC were selected for a transcriptional analysis in other stages of plant development, further confirming the signaling capacity of this molecule in plant physiology. Results from this analysis show that NO2-Ln application to 14- and 45-d-old arabidopsis plants followed the same trend both in up- and down-regulated genes, thus corroborating the signaling capacity of NO2-Ln throughout the overall development of arabidopsis [27].
An in-depth analysis of genes modulated by NO2-Ln indicated that up-regulated genes were involved mostly in protein folding and the response to heat and H2O2 [27]. In line with these results, a network graph analysis with GeneMANIA resource showed that around a 40% of the total genes induced by NO2-Ln treatment corresponded to members of the heat-shock response (HSR). Among these were numerous small heat-shock proteins (sHSP), but also some heat-shock transcription factors (HSFA2 and HSFA7B) as well as members of HSP40, HSP60, HSP70, and HSP90 families. The only transcriptomic analysis made to date with NO2-FAs in animal systems has been a microarray study with NO2-OA in human endothelial cell cultures [26] showing a noteworthy activation of the HSR in an Nrf2-independent manner. This activation of the HSP pathway contributes to cell protection through signaling actions involved in mainly anti-inflammatory processes. Outcomes derived from the study of the involvement of NO2-Ln in plant physiology resulted in a similar response to that previously observed with NO2-OA in animal systems, therefore suggesting a conserved mechanism of action of NO2-FAs both in animal and in plant systems. At this point, it is also important to bear in mind that incubation of ACSC with other NO2-FAs such as NO2-OA and NO2-LA generated a similar transcriptional response to that of the same subset of genes for NO2-Ln, suggesting a universality of the signaling mechanism that these molecules are able to trigger [27].
As mentioned above, NO2-Ln was involved in the response to several abiotic-stress conditions such as the response to heat, salinity, heavy metals, low temperature and mechanical wounding [27]. A general phenomenon underlying abiotic-stress situations is oxidative stress [68], [69]. Based on GO-term analysis, NO2-Ln has been associated with the response to H2O2, to reactive oxygen species (ROS), and to oxygen-containing compounds [27]. Among genes related to these processes, the high induction of cytosolic ascorbate peroxidase 2 (APX2) was noted together with other members involved in different redox processes [27]. APX is a key component of plant-defense responses against oxidative stress because this enzyme is able to detoxify the H2O2 generated under different stressful situations using ascorbate as an electron donor. Furthermore, the close interaction between the HSFA2 transcription factor and APX2 enzyme under stressful situations such as high temperature and light-intensity stress has been reported [70]. This nitro-fatty acid is able to act as a signaling mediator in the plant-defense mechanism in abiotic-stress situations, setting up a defense response against cell damage arising as a result of stress and mediated mainly by HSP induction. Therefore, these results suggest a close relationship with high temperature, abiotic stress in general, and oxidative stress—with both stress processes tightly regulated by NO2-Ln.
Finally, a high percentage of down-regulated genes were involved in the metabolism and decrease in sugar biosynthesis, in cell-wall components, and in chlorophyll, together with a decline in genes associated with photosynthesis, the electron-transport chain, and metabolite transport [27]. This phenomenon leads to a metabolic reconfiguration with a down-regulation in the biosynthesis of new components, counteracting energy costs and indicating is required to maintain a balance between the continuation of cell function and survival [71], [72].
4.2. NO2-Ln is a NO donor
All the results previously described show a significant involvement of NO2-Ln in plant physiology and specifically in the defense response to adverse abiotic conditions. However, the mechanisms by which this nitro-fatty acid is able to launch this plant-defense response remain unknown. As has been described for NO2-OA or NO2-LA in animal systems, the ability of NO2-Ln to modulate the generation of NO has also been assessed in the Arabidopsis in order to define how NO2-Ln sets up this antioxidant mechanism.
Firstly, several in vitro approaches were carried out to assess the capacity of NO2-Ln to generate NO. An initial study was performed using a spectrofluorometric assay with DAF-2 fluorophore in phosphate buffer and in the presence of 5 µM of NO2-Ln. Outcomes derived from this analysis showed that this molecule was able to boost fluorescence due to NO release, this being reduced with the application of the NO scavenger, cPTIO (2-(4-carboxyphenyl)−4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide) [35]. In this regard, a progressive increase in the NO release was registered up to 20 min and afterwards a decrease, this coinciding with a fall in the NO2-Ln content analyzed by HPLC-MS/MS [35]. In line with these results, the analysis of the ability of S-nitrosoglutathione (GSNO) to release NO has previously been described, and that GSNO has been shown to have a maximum of NO release at 30 min with a drastic reduction in this generation from this moment [73]. Therefore, both the biological NO donor GSNO and NO2-Ln have almost identical patterns of NO release in aqueous solutions. Due to pitfalls of NO detection with fluorophores such as DAF, the oxidation of oxyhemoglobin to methemoglobin due to NO release was also assessed with NO2-Ln. The incubation of this nitro-fatty acid with oxyhemoglobin led to typical changes in absorbance such as a decrease at 580 nm and 543 nm and a rise in the absorbance at 630 nm and 503 nm, confirming the ability of NO2-Ln to release NO by this approach [35]. Finally, this capacity was also quantified by ozone chemiluminescence such that both NO2-Ln (100 and 1000 µM) and NO2-LA (80 µM) reached a maximum of NO release at a neutral pH (pH 7.4) and a minimum at acidic conditions (pH 5.4 and 6.4) with an amount of NO release ranging from 10 to 210 nM/min [35]. These findings highlight that NO release from NO2-FAs is not favored under acidic conditions and this could have great relevance in cell compartments and organelles such as mitochondria, peroxisomes, and cytosol, where the pH values are neutral or basic [74].
Notably, although it has been proposed that NO release from NO2-FAs is less than 1% and is of minor significance in vivo [28], it might be worth considering that NO released.
in plant systems could have a significant importance due to the ability of NO2-Ln to release NO is 4.7–40-fold higher than this capacity analyzed in animal systems [35], which it is not surprising given that plant systems are the natural source of these fatty acids. Furthermore, it has been proposed that NO2-FA are not free in the cell under basal conditions, but part of cell membranes and/or adducted with proteins [14], [16]. In these situations, the ability to release NO is very low under physiological conditions. Nevertheless, the higher content of NO2-FAs detected under different abiotic stressful situations in plants such as wounding, low temperature, salinity or heavy metals [27], could occur with a parallel increase in the content of NO and may act as an important signaling mechanism under these adverse conditions.
On the other hand, it has been also shown that NO2-Ln is able to release NO in vivo. A preliminary study on the capacity of NO2-Ln to be a NO donor in vivo was performed in leaves and roots from 30-d-old arabidopsis plants pre-incubated with 1 mM NO2-Ln [75]. The result of this incubation significantly augmented green fluorescence due to NO release in both organs. Furthermore, the pre-treatment with cPTIO dramatically reduced fluorescence, thus confirming that NO2-Ln releases NO inside leaves and roots from 30-d-old arabidopsis plants. Afterwards, this ability was also checked in 7-d-old arabidopsis seedlings pre-incubated with 100 µM NO2-Ln, and a rise in fluorescence was also detected in roots from these plantlets and with a reduced fluorescence observed after cPTIO application [48]. Finally, with the use of 9-d-old ACSC, the same behavior resulted using 100 µM NO2-Ln [35]. In this respect, it is important to bear in mind that NO was found inside cells, where the pH is neutral. Accordingly, the capacity of NO2-Ln to be a NO donor seems to be important in organelles and cell compartments where the pH is neutral or basic. All these results underscore the capability of NO2-Ln to be a NO donor and the possible implications of this molecule in a wide range of signaling actions in plant physiology.
Fig. 1 shows a schematic model of NO2-Ln signaling. This nitro-fatty acid can establish a defense mechanism and an antioxidant response against adverse environmental conditions through the induction in the expression of a large set of heat-shock proteins (HSPs) and several antioxidant systems such as ascorbate peroxidase (APX), among others (pathway 1). Furthermore, it has recently been shown that NO2-Ln is a NO donor (pathway 2). Based on the key role of NO in a wide range of physiological and stress-response processes, the ability of NO2-Ln to act as a NO donor may have great relevance in plants. In fact, given that the content of NO2-Ln suffers a significant increase under several abiotic stress situations such as mechanical wounding, salinity, low temperature or cadmium stresses and the initial stage of Arabidopsis development [27], the NO release from increased NO2-Ln could be mediating diverse signaling actions that supporting these processes. Therefore, the ability of NO2-Ln to act as a NO donor may be able to support the NO implication in plant development, different biotic or abiotic processes, the antioxidant response or even mediating several post-translational modifications (NO-PTMs) as nitration and S-nitrosylation through which NO transmits its bioactivity [76], [77], [78]. In the latter case, NO released from NO2-Ln could bind to specific cysteine thiol groups of proteins thus promoting the formation of S-nitrosothiols (SNOs) and therefore be they implied in processes such as plant immunity [79], [80] or the response to abiotic stress conditions [21], [64], [81]. In fact, most of the S-nitrosylated proteins involved in the response to abiotic stress situations are redox-related proteins, such as ROS generating enzymes or antioxidant systems, suggesting a role of S-nitrosylation as a key regulator of the redox homeostasis during adverse environmental conditions. In this regard, results derived from RNA-seq analysis showed that ascorbate peroxidase is highly induced by NO2-Ln treatment [27]. Very recently, the S-nitrosylation of this enzyme was observed both in vitro and in vivo under salt stress and thus promoting this PTM an increase in the activity of this enzyme [61]. Therefore, NO released from NO2-Ln could be mediating an S-nitrosylation process of APX enzyme and hence increasing the enzyme activity, thus preventing the oxidative stress generated under this adverse condition. At this point, it could be important not to rule out that, based on the ability of NO2-Ln to be a NO donor, this molecule could be binding to free GSH on plants and generating S-nitrosoglutathione (GSNO), contributing to increase the levels of low molecular weight SNO in plant systems. Finally, NO derived from NO2-Ln could be reacting with superoxide anion (O2·-), commonly produced under several stress situations [69], thus generating the most powerful oxidant peroxynitrite (ONOO-) [82]. This RNS could be mediating the nitration of specific tyrosine residues from key cellular targets thus affecting concrete signaling pathways or, by contrast, provoking an increase in the protein nitration content.
The electrophilic capacity of nitroalkenes is also a feasible signaling pathway with important components of plant signaling (pathway 3). Results obtained in animal systems have shown that nitroalkylation of specific anti-inflammatory and antioxidant-related proteins leads to the modification of their activities, thus promoting important beneficial mechanisms [2], [7]. Due to there is no information available concerning this PTM in plants, analyzing this capacity with key components of antioxidant pathways could be useful to understand the mechanism of action of NO2-FAs in plant systems. In close relation to this, it is also plausible that under nitro-oxidative stress conditions, in which a rise in the content of ROS and RNS take place, the oxidation of the Michael adduct and subsequent release of free nitroalkenes could occur (pathway 4). This could have important signaling implications under stress conditions, because free NO2-FAs may be able to activate the relevant antioxidant properties that these molecules possess. Finally, the observed increase in the expression of APX could help alleviate the oxidative stress generated under stressful conditions by detoxifying the H2O2 produced under these circumstances.
5. Conclusions and future perspectives
A significant advance in understanding the interaction between NO and NO-derived molecules with unsaturated fatty acids has been achieved in animal systems. This interaction has started to be studied very recently in plant systems with the endogenous occurrence of nitrated derivatives of linolenic acid in the model plant arabidopsis. Although an antioxidant response and a defense mechanism against several abiotic-stress conditions is activated by NO2-Ln and the ability of this molecule to release NO has also been reported, the precise mechanisms by which this nitro-fatty acid is able to induce these responses remain unknown. In this regard, future research should be focused on analyzing the electrophilic ability of NO2-FAs with specific plant-cell targets and their involvement in plant signaling. It is also important to take into account the capacity of NO2-Ln to release NO as well as the further implications of this molecule in a wide range of actions in plant physiology. Moreover, other mechanisms involving NO2-FAs could be considered which will hopefully offer insight into how NO2-Ln signaling regulates the important antioxidant and defense mechanisms that this molecule is able to set up. Finally, current mass spectrometry approaches have achieved a higher sensitivity allowing the routine and suitable level detection of these important signaling molecules. Therefore and in view of this landscape, NO2-FAs could be considered as new key mediators of NO metabolism in both physiological and stress conditions.
Acknowledgments
CM-P would like to thank the University of Jaén for funding the Ph.D. fellowship. This study was supported by the ERDF grants co-financed by the Ministry of Economy and Competitiveness (projects BIO2015-66390-P and AGL2015-65104-P) and the Junta de Andalucía (groups BIO286 and BIO192) in Spain.
References
- 1.Rudolph V., Rudolph T.K., Schopfer F.J., Bonacci G., Woodcock S.R., Cole M.P., Baker P.R., Ramani R., Freeman B.A. Endogenous generation and protective effects of nitro-fatty acids in a murine model of focal cardiac ischaemia and reperfusion. Cardiovasc. Res. 2010;85:155–166. doi: 10.1093/cvr/cvp275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schopfer F.J., Cipollina C., Freeman B.A. Formation and signaling actions of electrophilic lipids. Chem. Rev. 2011;111:5997–6021. doi: 10.1021/cr200131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker P.R., Schopfer F.J., O’Donnell V.B., Freeman B.A. Convergence of nitric oxide and lipid signaling: anti-inflammatory nitro-fatty acids. Free Radic. Biol. Med. 2009;46:989–1003. doi: 10.1016/j.freeradbiomed.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lima É.S., Di Mascio P., Rubbo H., Abdalla D.S. Characterization of linoleic acid nitration in human blood plasma by mass spectrometry. Biochemistry. 2002;41:10717–10722. doi: 10.1021/bi025504j. [DOI] [PubMed] [Google Scholar]
- 5.Bonacci G., Baker P.R., Salvatore S.R., Shores D., Khoo N.K., Koenitzer J.R., Vitturi D.A., Woodcock S.R., Golin-Bisello F., Cole M.P. Conjugated linoleic acid is a preferential substrate for fatty acid nitration. J. Biol. Chem. 2012;287:44071–44082. doi: 10.1074/jbc.M112.401356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villacorta L., Zhang J., Garcia-Barrio M.T., Chen X.-l., Freeman B.A., Chen Y.E., Cui T. Vol. 293. 2007. Nitro-linoleic acid inhibits vascular smooth muscle cell proliferation via the Keap1/Nrf2 signaling pathway; pp. H770–H776. (Am. J. Physiol.-Heart Circ. Physiol.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villacorta L., Gao Z., Schopfer F.J., Freeman B.A., Chen Y.E. Nitro-fatty acids in cardiovascular regulation and diseases: characteristics and molecular mechanisms. Front. Biosci. (Landmark Ed.) 2015;21:873–889. doi: 10.2741/4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambrozova G., Fidlerova T., Verescakova H., Koudelka A., Rudolph T.K., Woodcock S.R., Freeman B.A., Kubala L., Pekarova M. Nitro-oleic acid inhibits vascular endothelial inflammatory responses and the endothelial-mesenchymal transition. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2016;1860:2428–2437. doi: 10.1016/j.bbagen.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelley E.E., Batthyany C.I., Hundley N.J., Woodcock S.R., Bonacci G., Del Rio J.M., Schopfer F.J., Lancaster J.R., Freeman B.A., Tarpey M.M. Nitro-oleic acid, a novel and irreversible inhibitor of xanthine oxidoreductase. J. Biol. Chem. 2008;283:36176–36184. doi: 10.1074/jbc.M802402200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitturi D.A., Minarrieta L., Salvatore S.R., Postlethwait E.M., Fazzari M., Ferrer-Sueta G., Lancaster J.R., Jr, Freeman B.A., Schopfer F.J. Convergence of biological nitration and nitrosation via symmetrical nitrous anhydride. Nat. Chem. Biol. 2015;11:504–510. doi: 10.1038/nchembio.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeman B.A., Baker P.R., Schopfer F.J., Woodcock S.R., Napolitano A., d'Ischia M. Nitro-fatty acid formation and signaling. J. Biol. Chem. 2008 doi: 10.1074/jbc.R800004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vitturi D.A., Chen C.-S., Woodcock S.R., Salvatore S.R., Bonacci G., Koenitzer J.R., Stewart N.A., Wakabayashi N., Kensler T.W., Freeman B.A. Modulation of nitro-fatty acid signaling prostaglandin reductase-1 is a nitroalkene reductase. J. Biol. Chem. 2013;288:25626–25637. doi: 10.1074/jbc.M113.486282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salvatore S.R., Vitturi D.A., Baker P.R., Bonacci G., Koenitzer J.R., Woodcock S.R., Freeman B.A., Schopfer F.J. Characterization and quantification of endogenous fatty acid nitroalkene metabolites in human urine. J. Lipid Res. 2013;54:1998–2009. doi: 10.1194/jlr.M037804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubbo H., Trostchansky A. Springer; 2014. Nitro-fatty Acids: Synthesis, Properties, And Role in Biological System, Nitric Oxide in Plants: metabolism and Role in Stress Physiology; pp. 153–162. [Google Scholar]
- 15.Trostchansky A., Rubbo H. Nitrated fatty acids: mechanisms of formation, chemical characterization, and biological properties. Free Radic. Biol. Med. 2008;44:1887–1896. doi: 10.1016/j.freeradbiomed.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Schopfer F., Batthyany C., Baker P., Bonacci G., Cole M., Rudolph V., Groeger A., Rudolph T., Nadtochiy S., Brookes P. Detection and quantification of protein adduction by electrophilic fatty acids: mitochondrial generation of fatty acid nitroalkene derivatives. Free Radic. Biol. Med. 2009;46:1250–1259. doi: 10.1016/j.freeradbiomed.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Padilla M.N., Mata-Pérez C., Melguizo M., Barroso J. In vitro nitro-fatty acid release from Cys-NO2-fatty acid adducts under nitro-oxidative conditions. Nitric Oxide. 2016 doi: 10.1016/j.niox.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brieger K., Schiavone S., Miller F.J., Jr, Krause K.-H. Reactive oxygen species: from health to disease. Swiss Med. Wkly. 2012;142:w13659. doi: 10.4414/smw.2012.13659. [DOI] [PubMed] [Google Scholar]
- 20.Corpas F.J., Chaki M., Leterrier M., Barroso J.B. Protein tyrosine nitration: a new challenge in plants. Plant Signal. Behav. 2009;4:920–923. doi: 10.4161/psb.4.10.9466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaki M., Valderrama R., Fernández-Ocaña A.M., Carreras A., Gómez-Rodríguez M.V., Pedrajas J.R., Begara-Morales J.C., Sánchez-Calvo B., Luque F., Leterrier M. Mechanical wounding induces a nitrosative stress by down-regulation of GSNO reductase and an increase in S-nitrosothiols in sunflower (Helianthus annuus) seedlings. J. Exp. Bot. 2011;62:1803–1813. doi: 10.1093/jxb/erq358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Begara-Morales J.C., Chaki M., Sánchez-Calvo B., Mata-Pérez C., Leterrier M., Palma J.M., Barroso J.B., Corpas F.J. Protein tyrosine nitration in pea roots during development and senescence. J. Exp. Bot. 2013;64:1121–1134. doi: 10.1093/jxb/ert006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui T., Schopfer F.J., Zhang J., Chen K., Ichikawa T., Baker P.R., Batthyany C., Chacko B.K., Feng X., Patel R.P. Nitrated fatty acids: endogenous anti-inflammatory signaling mediators. J. Biol. Chem. 2006;281:35686–35698. doi: 10.1074/jbc.M603357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole M.P., Rudolph T.K., Khoo N.K., Motanya U.N., Golin-Bisello F., Wertz J.W., Schopfer F.J., Rudolph V., Woodcock S.R., Bolisetty S. Nitro-fatty acid inhibition of neointima formation after endoluminal vessel injury. Circ. Res. 2009;105:965–972. doi: 10.1161/CIRCRESAHA.109.199075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferreira A., Ferrari M., Trostchansky A., Batthyany C., Souza J., Alvarez M., Lopez G., Baker P., Schopfer F., O'Donnell V. Macrophage activation induces formation of the anti-inflammatory lipid cholesteryl-nitrolinoleate. Biochem. J. 2009;417:223–234. doi: 10.1042/BJ20080701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kansanen E., Jyrkkänen H.-K., Volger O.L., Leinonen H., Kivelä A.M., Häkkinen S.-K., Woodcock S.R., Schopfer F.J., Horrevoets A.J., Ylä-Herttuala S. Nrf2-dependent and-independent responses to nitro-fatty acids in human endothelial cells identification of heat shock response as the major pathway activated by nitro-oleic acid. J. Biol. Chem. 2009;284:33233–33241. doi: 10.1074/jbc.M109.064873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mata-Pérez C., Sánchez-Calvo B., de las Nieves Padilla-Serrano M., Begara-Morales J.C., Luque F., Melguizo M., Jiménez-Ruiz J., Fierro-Risco J., Peñas-Sanjuán A., Valderrama R. Nitro-fatty acids in plant signaling: nitro-linolenic acid induces the molecular chaperone network in Arabidopsis. Plant Physiol. 2016;170:686–701. doi: 10.1104/pp.15.01671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geisler A.C., Rudolph T.K. Nitroalkylation—a redox sensitive signaling pathway. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2012;1820:777–784. doi: 10.1016/j.bbagen.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Delmastro-Greenwood M., Freeman B.A., Wendell S.G. Redox-dependent anti-inflammatory signaling actions of unsaturated fatty acids. Annu. Rev. Physiol. 2014;76:79. doi: 10.1146/annurev-physiol-021113-170341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schopfer F.J., Lin Y., Baker P.R., Cui T., Garcia-Barrio M., Zhang J., Chen K., Chen Y.E., Freeman B.A. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor γ ligand. Proc. Natl. Acad. Sci. USA. 2005;102:2340–2345. doi: 10.1073/pnas.0408384102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y., Zhang J., Schopfer F.J., Martynowski D., Garcia-Barrio M.T., Kovach A., Suino-Powell K., Baker P.R., Freeman B.A., Chen Y.E. Molecular recognition of nitrated fatty acids by PPARγ. Nat. Struct. Mol. Biol. 2008;15:865–867. doi: 10.1038/nsmb.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kansanen E., Bonacci G., Schopfer F.J., Kuosmanen S.M., Tong K.I., Leinonen H., Woodcock S.R., Yamamoto M., Carlberg C., Ylä-Herttuala S. Electrophilic nitro-fatty acids activate NRF2 by a KEAP1 cysteine 151-independent mechanism. J. Biol. Chem. 2011;286:14019–14027. doi: 10.1074/jbc.M110.190710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorczynski M.J., Huang J., Lee H., King S.B. Evaluation of nitroalkenes as nitric oxide donors. Bioorg. Med. Chem. Lett. 2007;17:2013–2017. doi: 10.1016/j.bmcl.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Schopfer F.J., Baker P.R., Giles G., Chumley P., Batthyany C., Crawford J., Patel R.P., Hogg N., Branchaud B.P., Lancaster J.R. Fatty acid transduction of nitric oxide signaling Nitrolinoleic acid is a hydrophobically stabilized nitric oxide donor. J. Biol. Chem. 2005;280:19289–19297. doi: 10.1074/jbc.M414689200. [DOI] [PubMed] [Google Scholar]
- 35.Mata-Pérez C., Sánchez-Calvo B., Begara-Morales J.C., Carreras A., Padilla M.N., Melguizo M., Valderrama R., Corpas F.J., Barroso J.B. Nitro-linolenic acid is a nitric oxide donor. Nitric Oxide. 2016;57:57–63. doi: 10.1016/j.niox.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Lima É.S., Bonini M.G., Augusto O., Barbeiro H.V., Souza H.P., Abdalla D.S. Nitrated lipids decompose to nitric oxide and lipid radicals and cause vasorelaxation. Free Radic. Biol. Med. 2005;39:532–539. doi: 10.1016/j.freeradbiomed.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Baker L.M., Baker P.R., Golin-Bisello F., Schopfer F.J., Fink M., Woodcock S.R., Branchaud B.P., Radi R., Freeman B.A. Nitro-fatty acid reaction with glutathione and cysteine Kinetic analysis of thiol alkylation by a Michael addition reaction. J. Biol. Chem. 2007;282:31085–31093. doi: 10.1074/jbc.M704085200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batthyany C., Schopfer F.J., Baker P.R., Durán R., Baker L.M., Huang Y., Cerveñansky C., Branchaud B.P., Freeman B.A. Reversible post-translational modification of proteins by nitrated fatty acids in vivo. J. Biol. Chem. 2006;281:20450–20463. doi: 10.1074/jbc.M602814200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faine L.A., Cavalcanti D.M., Rudnicki M., Ferderbar S., Macedo S., Souza H.P., Farsky S.H., Boscá L., Abdalla D.S.P. Bioactivity of nitrolinoleate: effects on adhesion molecules and CD40–CD40L system. J. Nutr. Biochem. 2010;21:125–132. doi: 10.1016/j.jnutbio.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Esposito K., Marfella R., Ciotola M., Di Palo C., Giugliano F., Giugliano G., D'Armiento M., D'Andrea F., Giugliano D. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. Jama. 2004;292:1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 41.Carluccio M.A., Siculella L., Ancora M.A., Massaro M., Scoditti E., Storelli C., Visioli F., Distante A., De Caterina R. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation antiatherogenic properties of mediterranean diet phytochemicals. Arterioscler., Thromb., Vasc. Biol. 2003;23:622–629. doi: 10.1161/01.ATV.0000062884.69432.A0. [DOI] [PubMed] [Google Scholar]
- 42.Covas M.-I. Olive oil and the cardiovascular system. Pharmacol. Res. 2007;55:175–186. doi: 10.1016/j.phrs.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Fazzari M., Trostchansky A., Schopfer F.J., Salvatore S.R., Sánchez-Calvo B., Vitturi D., Valderrama R., Barroso J.B., Radi R., Freeman B.A., Rubbo H. Olives and olive oil are sources of electrophilic fatty acid nitroalkenes. PloS One. 2014;9:e84884. doi: 10.1371/journal.pone.0084884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coles B., Bloodsworth A., Clark S.R., Lewis M.J., Cross A.R., Freeman B.A., O’Donnell V.B. Nitrolinoleate inhibits superoxide generation, degranulation, and integrin expression by human neutrophils novel antiinflammatory properties of nitric oxide–derived reactive species in vascular cells. Circ. Res. 2002;91:375–381. doi: 10.1161/01.res.0000032114.68919.ef. [DOI] [PubMed] [Google Scholar]
- 45.Schopfer F.J., Cole M.P., Groeger A.L., Chen C.-S., Khoo N.K., Woodcock S.R., Golin-Bisello F., Motanya U.N., Li Y., Zhang J. Covalent peroxisome proliferator-activated receptor γ adduction by nitro-fatty acids selective ligand activity and anti-diabetic signaling actions. J. Biol. Chem. 2010;285:12321–12333. doi: 10.1074/jbc.M109.091512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wright M.M., Schopfer F.J., Baker P.R., Vidyasagar V., Powell P., Chumley P., Iles K.E., Freeman B.A., Agarwal A. Fatty acid transduction of nitric oxide signaling: nitrolinoleic acid potently activates endothelial heme oxygenase 1 expression. Proc. Natl. Acad. Sci. USA. 2006;103:4299–4304. doi: 10.1073/pnas.0506541103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsikas D., Zoerner A.A., Mitschke A., Gutzki F.-M. Nitro-fatty acids occur in human plasma in the picomolar range: a targeted nitro-lipidomics GC–MS/MS study. Lipids. 2009;44:855–865. doi: 10.1007/s11745-009-3332-4. [DOI] [PubMed] [Google Scholar]
- 48.Mata-Pérez C., Sánchez-Calvo B., Begara-Morales J.C., Padilla M.N., Valderrama R., Corpas F.J., Barroso J.B. Nitric oxide release from nitro-fatty acids in Arabidopsis roots. Plant Signal. Behav. 2016 doi: 10.1080/15592324.2016.1154255. (00-00) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beligni M.V., Lamattina L. Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta. 2000;210:215–221. doi: 10.1007/PL00008128. [DOI] [PubMed] [Google Scholar]
- 50.Albertos P., Romero-Puertas M.C., Tatematsu K., Mateos I., Sánchez-Vicente I., Nambara E., Lorenzo O. S-nitrosylation triggers ABI5 degradation to promote seed germination and seedling growth. Nat. Commun. 2015;6 doi: 10.1038/ncomms9669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beligni Ma.V., Lamattina L. Nitric oxide: a non-traditional regulator of plant growth. Trends Plant Sci. 2001;6:508–509. doi: 10.1016/s1360-1385(01)02156-2. [DOI] [PubMed] [Google Scholar]
- 52.Ahlfors R., Brosché M., Kollist H., Kangasjärvi J. Nitric oxide modulates ozone‐induced cell death, hormone biosynthesis and gene expression in Arabidopsis thaliana. Plant J. 2009;58:1–12. doi: 10.1111/j.1365-313X.2008.03756.x. [DOI] [PubMed] [Google Scholar]
- 53.Besson-Bard A., Astier J., Rasul S., Wawer I., Dubreuil-Maurizi C., Jeandroz S., Wendehenne D. Current view of nitric oxide-responsive genes in plants. Plant Sci. 2009;177:302–309. [Google Scholar]
- 54.Bethke P.C., Libourel I.G., Jones R.L. Nitric oxide reduces seed dormancy in Arabidopsis. J. Exp. Bot. 2006;57:517–526. doi: 10.1093/jxb/erj060. [DOI] [PubMed] [Google Scholar]
- 55.Corpas F.J., Barroso J.B., Carreras A., Quirós M., León A.M., Romero-Puertas M.C., Esteban F.J., Valderrama R., Palma J.M., Sandalio L.M. Cellular and subcellular localization of endogenous nitric oxide in young and senescent pea plants. Plant Physiol. 2004;136:2722–2733. doi: 10.1104/pp.104.042812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Correa-Aragunde N., Graziano M., Chevalier C., Lamattina L. Nitric oxide modulates the expression of cell cycle regulatory genes during lateral root formation in tomato. J. Exp. Bot. 2006;57:581–588. doi: 10.1093/jxb/erj045. [DOI] [PubMed] [Google Scholar]
- 57.de Pinto M.C., Locato V., Sgobba A., del Carmen Romero-Puertas M., Gadaleta C., Delledonne M., De Gara L. S-nitrosylation of ascorbate peroxidase is part of programmed cell death signaling in tobacco Bright Yellow-2 cells. Plant Physiol. 2013;163:1766–1775. doi: 10.1104/pp.113.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Delledonne M., Xia Y., Dixon R.A., Lamb C. Nitric oxide functions as a signal in plant disease resistance. Nature. 1998;394:585–588. doi: 10.1038/29087. [DOI] [PubMed] [Google Scholar]
- 59.Delledonne M., Zeier J., Marocco A., Lamb C. Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc. Natl. Acad. Sci. 2001;98:13454–13459. doi: 10.1073/pnas.231178298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gaupels F., Spiazzi-Vandelle E., Yang D., Delledonne M. Detection of peroxynitrite accumulation in Arabidopsis thaliana during the hypersensitive defense response. Nitric Oxide. 2011;25:222–228. doi: 10.1016/j.niox.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 61.Begara-Morales J.C., Sánchez-Calvo B., Chaki M., Valderrama R., Mata-Pérez C., López-Jaramillo J., Padilla M.N., Carreras A., Corpas F.J., Barroso J.B. Dual regulation of cytosolic ascorbate peroxidase (APX) by tyrosine nitration and S-nitrosylation. J. Exp. Bot. 2014:ert396. doi: 10.1093/jxb/ert396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chaki M., Carreras A., López-Jaramillo J., Begara-Morales J.C., Sánchez-Calvo B., Valderrama R., Corpas F.J., Barroso J.B. Tyrosine nitration provokes inhibition of sunflower carbonic anhydrase (β-CA) activity under high temperature stress. Nitric Oxide. 2013;29:30–33. doi: 10.1016/j.niox.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 63.Chaki M., Fernández-Ocaña A.M., Valderrama R., Carreras A., Esteban F.J., Luque F., Gómez-Rodríguez M.V., Begara-Morales J.C., Corpas F.J., Barroso J.B. Involvement of reactive nitrogen and oxygen species (RNS and ROS) in sunflower–mildew interaction. Plant Cell Physiol. 2009;50:265–279. doi: 10.1093/pcp/pcn196. [DOI] [PubMed] [Google Scholar]
- 64.Chaki M., Valderrama R., Fernández‐Ocaña A.M., Carreras A., Gómez‐Rodríguez M.V., López‐Jaramillo J., Begara‐Morales J.C., Sánchez‐Calvo B., Luque F., Leterrier M. High temperature triggers the metabolism of S‐nitrosothiols in sunflower mediating a process of nitrosative stress which provokes the inhibition of ferredoxin–NADP reductase by tyrosine nitration. Plant, Cell Environ. 2011;34:1803–1818. doi: 10.1111/j.1365-3040.2011.02376.x. [DOI] [PubMed] [Google Scholar]
- 65.Airaki M., Leterrier M., Mateos R.M., Valderrama R., Chaki M., Barroso J.B., Del Rio L.A., Palma J.M., Corpas F.J. Metabolism of reactive oxygen species and reactive nitrogen species in pepper (Capsicum annuum L.) plants under low temperature stress. Plant, Cell Environ. 2012;35:281–295. doi: 10.1111/j.1365-3040.2011.02310.x. [DOI] [PubMed] [Google Scholar]
- 66.Corpas F.J., Chaki M., Fernández-Ocaña A., Valderrama R., Palma J.M., Carreras A., Begara-Morales J.C., Airaki M., del Río L.A., Barroso J.B. Metabolism of reactive nitrogen species in pea plants under abiotic stress conditions. Plant Cell Physiol. 2008;49:1711–1722. doi: 10.1093/pcp/pcn144. [DOI] [PubMed] [Google Scholar]
- 67.Blanquet P., Silva L., Catrice O., Bruand C., Carvalho H., Meilhoc E. Sinorhizobium meliloti controls nitric oxide–mediated post-translational modification of a Medicago truncatula nodule protein. Mol. Plant-Microbe Interact. 2015;28:1353–1363. doi: 10.1094/MPMI-05-15-0118-R. [DOI] [PubMed] [Google Scholar]
- 68.Apel K., Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 69.Miller G., Shulaev V., Mittler R. Reactive oxygen signaling and abiotic stress. Physiol. Plant. 2008;133:481–489. doi: 10.1111/j.1399-3054.2008.01090.x. [DOI] [PubMed] [Google Scholar]
- 70.Nishizawa A., Yabuta Y., Yoshida E., Maruta T., Yoshimura K., Shigeoka S. Arabidopsis heat shock transcription factor A2 as a key regulator in response to several types of environmental stress. Plant J. 2006;48:535–547. doi: 10.1111/j.1365-313X.2006.02889.x. [DOI] [PubMed] [Google Scholar]
- 71.Fahnenstich H., Scarpeci T.E., Valle E.M., Flügge U.-I., Maurino V.G. Generation of hydrogen peroxide in chloroplasts of Arabidopsis overexpressing glycolate oxidase as an inducible system to study oxidative stress. Plant Physiol. 2008;148:719–729. doi: 10.1104/pp.108.126789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mahajan N.S., Mishra M., Tamhane V.A., Gupta V.S., Giri A.P. Stress inducible proteomic changes in Capsicum annuum leaves. Plant Physiol. Biochem. 2014;74:212–217. doi: 10.1016/j.plaphy.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 73.Ederli L., Reale L., Madeo L., Ferranti F., Gehring C., Fornaciari M., Romano B., Pasqualini S. NO release by nitric oxide donors in vitro and in planta. Plant Physiol. Biochem. 2009;47:42–48. doi: 10.1016/j.plaphy.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 74.Shen J., Zeng Y., Zhuang X., Sun L., Yao X., Pimpl P., Jiang L. Organelle pH in the Arabidopsis endomembrane system. Mol. Plant. 2013;6:1419–1437. doi: 10.1093/mp/sst079. [DOI] [PubMed] [Google Scholar]
- 75.Sánchez-Calvo B., Barroso J.B., Corpas F.J. Hypothesis: nitro-fatty acids play a role in plant metabolism. Plant Sci. 2013;199:1–6. doi: 10.1016/j.plantsci.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 76.Benhar M., Forrester M.T., Hess D.T., Stamler J.S. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science. 2008;320:1050–1054. doi: 10.1126/science.1158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kneeshaw S., Gelineau S., Tada Y., Loake G.J., Spoel S.H. Selective Protein denitrosylation activity of Thioredoxin-h5 modulates plant immunity. Mol. Cell. 2014;56:153–162. doi: 10.1016/j.molcel.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 78.Hess D.T., Matsumoto A., Kim S.-O., Marshall H.E., Stamler J.S. Protein S-nitrosylation: purview and parameters. Nat. Rev. Mol. Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 79.Feechan A., Kwon E., Yun B.-W., Wang Y., Pallas J.A., Loake G.J. A central role for S-nitrosothiols in plant disease resistance. Proc. Natl. Acad. Sci. 2005;102:8054–8059. doi: 10.1073/pnas.0501456102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yun B.W., Skelly M.J., Yin M., Yu M., Mun B.G., Lee S.U., Hussain A., Spoel S.H., Loake G.J. Nitric oxide and S‐nitrosoglutathione function additively during plant immunity. New Phytol. 2016 doi: 10.1111/nph.13903. [DOI] [PubMed] [Google Scholar]
- 81.Corpas F.J., Chaki M., Begara-Morales J.C., Valderrama R., Sánchez-Calvo B., Barroso J.B. Functional implications of S-nitrosothiols under nitrooxidative stress induced by abiotic conditions. Adv. Bot. Res. 2016 [Google Scholar]
- 82.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc. Natl. Acad. Sci. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]