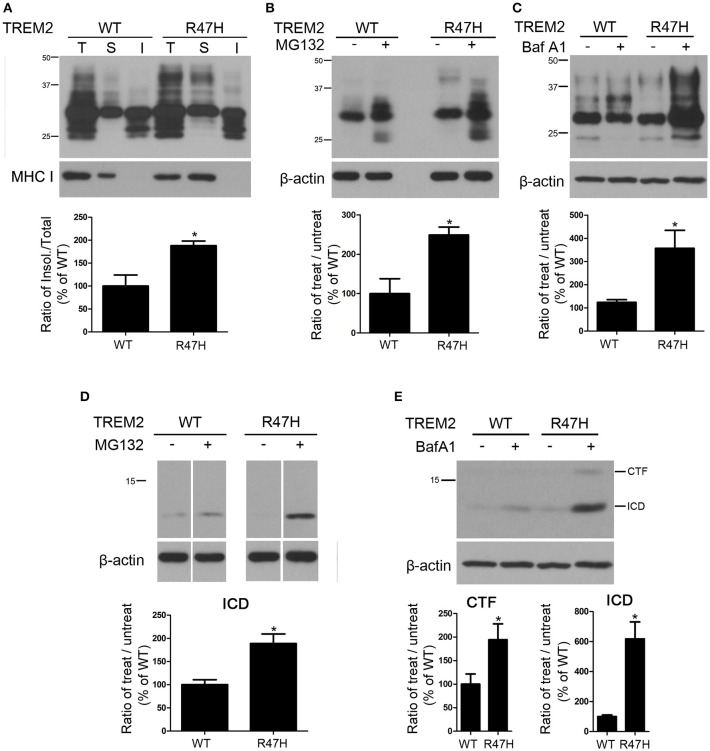
Figure 4.
Decreased solubility of TREM2 R47H. (A) Protein samples from HeLa cells expressing wild-type TREM2 and its R47H variant were fractionated with detergent. TREM2 R47H was found to be increased in the detergent-insoluble fractions. Major histocompatibility complex (MHC)-I was used as a loading control for verification of fractionation. (T, total fraction; S, soluble fraction; I, insoluble fraction). (B) HeLa cells expressing wild-type TREM2 and its R47H variant were treated with MG 132 (10 μM) for 16 h. The TREM2 R47H levels were elevated compared to wild-type. (C) Following bafilomycin A1 (Baf A1) (25 nM) treatment for 16 h, TREM2 R47H expression was increased compared to wild-type. (D,E) The intracellular domain (ICD) bands of TREM2 are increased by treatment with MG132. Cropped images from the original blot (Supplementary Figure 2) are displayed here. Following bafilomycin A1 treatment, the CTF and ICD bands of TREM2 R47H are increased compared to wild-type. These data represent the mean ± S.E. from three independent experiments. *P < 0.05.